Lafutidine tablet and preparation method thereof
A technology for lafutidine tablets and futidine tablets, which is applied in the field of drug preparation, can solve the problems of dissimilar dissolution curves, poor stability and the like, achieves low toxicity and side effects, high stability, and ensures safety and effectiveness Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0012] A preparation method for lafutidine tablet, comprising the following steps:
[0013] S1, lafutidine raw material pretreatment;
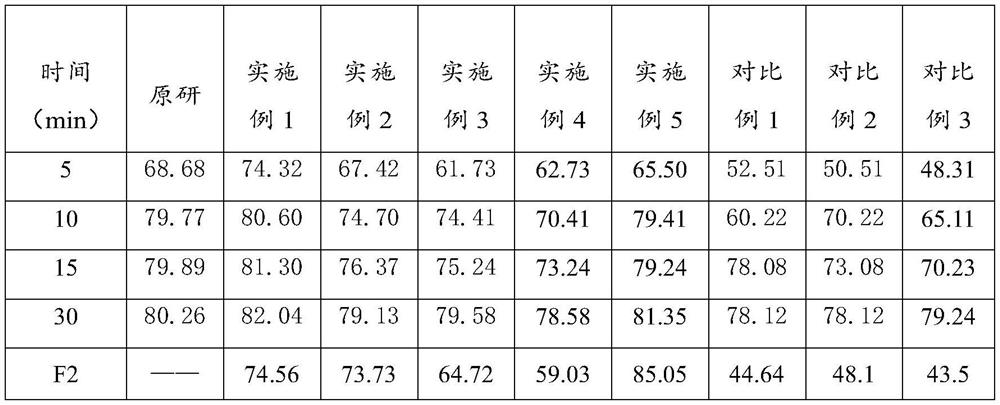
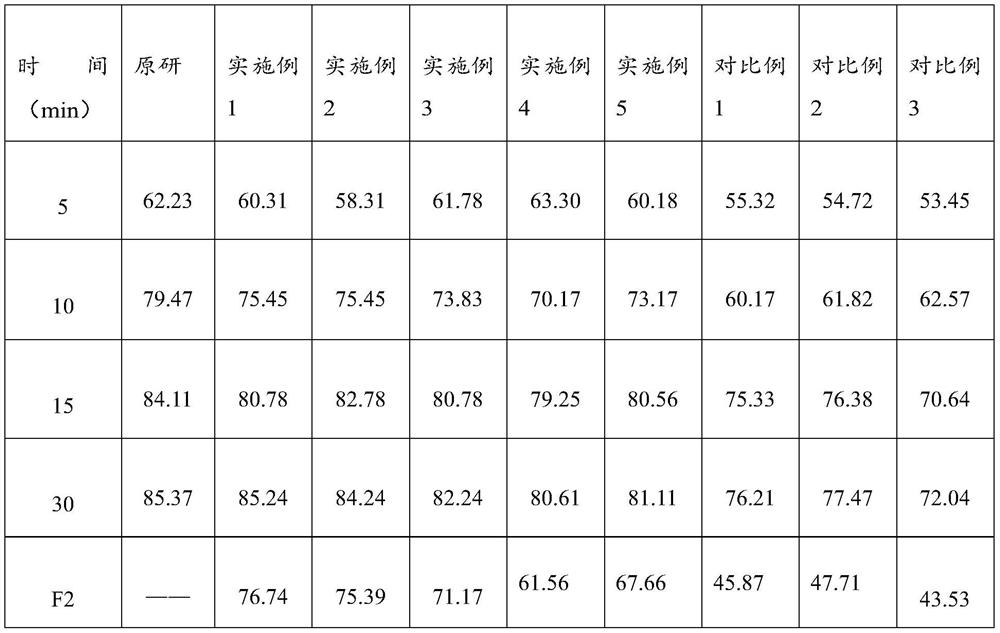
[0014] The lafutidine raw material is pretreated so that the D10 of the pretreated lafutidine raw material is 1.015-2.16 μm; the D50 is 2.684-7.367 μm; and the D90 is 4.325-52.8 μm. In the prior art, the lafutidine tablet has poor stability, and the dissolution effect is not good. The inventors found that it is because the lafutidine raw material has a large electrostatic effect, and it is easy to agglomerate together, and then it is easy to cause uneven content of the main drug, which affects stability. Sexuality also leads to poor dissolution effect. The inventors found that by controlling the particle size of the lafutidine raw material, the aggregation of the lafutidine raw material can be effectively controlled to ensure that the prepared lafutidine tablet has good stability and dissolution effect. If during screening, the selected mesh...
Embodiment 1
[0043] The present embodiment provides a kind of preparation method of lafutidine tablet, comprises the following steps:
[0044] S1. Lafutidine raw material pretreatment——pass 50g of lafutidine raw material through a 120-mesh sieve, and after pretreatment, lafutidine raw material D 10 2.16μm, D 50 7.367μm, D 90 is 52.8 μm.
[0045] S2, preparing the main drug-containing granules;
[0046] 23.40 g of hydroxypropyl cellulose was dissolved in 444.6 g of water to obtain a binder with a concentration of 5% by mass.
[0047] The sieved lafutidine raw material was mixed with 14.98 g of corn starch, 319.64 g of lactose, 125.39 g of microcrystalline cellulose and 29.7 g of croscarmellose sodium to obtain a dry-blended material. Then the dry-blended material is mixed with the above-mentioned binder and sheared for 60 seconds, then granulated, and then dried and granulated through a 24-mesh sieve to obtain main drug-containing granules. The drying adopts fluidized bed drying, and t...
Embodiment 2-5
[0052] The preparation method of the lafutidine tablet provided by embodiment 2-5 is basically the same as the preparation method of the lafutidine tablet provided by embodiment 1. The difference is the specific operation of the preparation method provided by embodiment 2-5. Conditions vary and specific substances used vary.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com