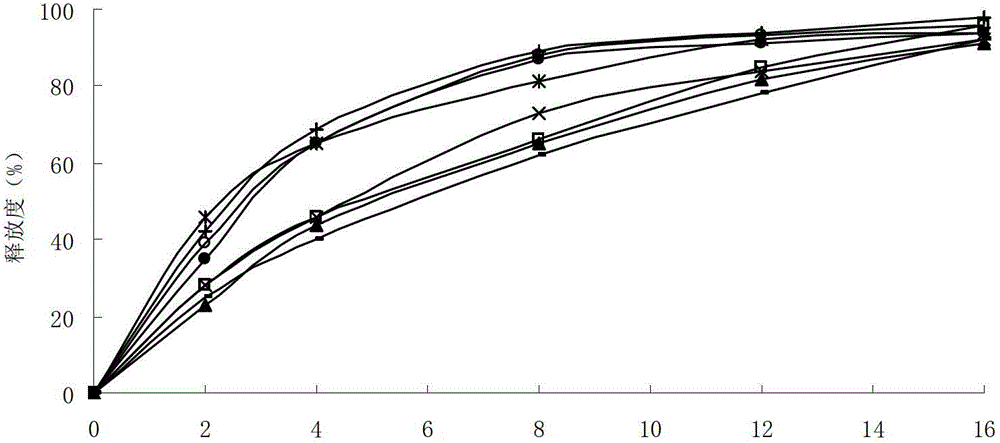
Lafutidine liposome solid preparation and preparing method thereof
A technology for lafutidine and futidine is applied in the field of medicine to achieve the effects of high product quality, long retention time and significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Preparation of Lafutidine Liposome Tablets
[0070]
[0071]
[0072] Adopt following production process to prepare lafutidine liposome tablet:
[0073] (1) Dissolve 5g of lafutidine, 125g of dipalmitoylphosphatidylglycerol, 125g of sodium deoxycholate and 50g of Span 80 in 2000ml of methanol, and stir to dissolve;
[0074] (2) Put the above solution in an eggplant-shaped bottle, remove methanol under reduced pressure in a water bath at 45°C, and form a uniform transparent film on the wall of the bottle;
[0075] (3) Add 2000ml of acetate buffer solution with a pH value of 6.2 to the eggplant-shaped bottle, and continue to rotate in a water bath at 45°C under normal pressure to swell and hydrate the film;
[0076] (4) Filter the above solution with a 0.45 μm microporous membrane, place the filtrate in a refrigerator at -20°C to freeze overnight, then take it out and thaw it, freeze and thaw repeatedly three times, and spray dry to obtain lafutidine li...
Embodiment 2
[0080] Example 2 Preparation of Lafutidine Liposome Capsules
[0081]
[0082] Adopt following production process to prepare lafutidine liposome capsule:
[0083] (1) Dissolve 10g of lafutidine, 300g of dipalmitoylphosphatidylglycerol, 150g of sodium deoxycholate and 80g of Span 80 in 2000ml of methanol, and stir to dissolve;
[0084] (2) Put the above solution in an eggplant-shaped bottle, remove methanol under reduced pressure in a water bath at 45°C, and form a uniform transparent film on the wall of the bottle;
[0085] (3) Add 2000ml of acetate buffer solution with a pH value of 6.2 to the eggplant-shaped bottle, and continue to rotate in a water bath at 45°C under normal pressure to swell and hydrate the film;
[0086] (4) Filter the above solution with a 0.45 μm microporous membrane, freeze the filtrate overnight in a -20°C refrigerator, take it out and thaw it, freeze and thaw repeatedly three times, and spray dry to obtain lafutidine liposome powder.
[0087] (...
Embodiment 3
[0090] Example 3 Preparation of Lafutidine Liposome Dispersible Tablets
[0091]
[0092] Adopt following production process to prepare lafutidine liposome dispersible tablet:
[0093] (1) Dissolve 5g of lafutidine, 150g of dipalmitoylphosphatidylglycerol, 100g of sodium deoxycholate and 100g of Span 80 in 2000ml of methanol, and stir to dissolve;
[0094] (2) Put the above solution in an eggplant-shaped bottle, remove methanol under reduced pressure in a water bath at 45°C, and form a uniform transparent film on the wall of the bottle;
[0095] (3) Add 2000ml of acetate buffer solution with a pH value of 6.2 to the eggplant-shaped bottle, and continue to rotate in a water bath at 45°C under normal pressure to swell and hydrate the film;
[0096] (4) Filter the above solution with a 0.45 μm microporous membrane, freeze the filtrate overnight in a -20°C refrigerator, take it out and thaw it, freeze and thaw repeatedly three times, and spray dry to obtain lafutidine liposo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com