Novel technology for preparing lafutidine
A technology of lafutidine and butene, which is applied in the field of medicine and biology, can solve the problems of unfavorable large-scale industrial production, high cost of lafutidine, and low yield, and achieve easy operation, low reaction cost, and mild reaction Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
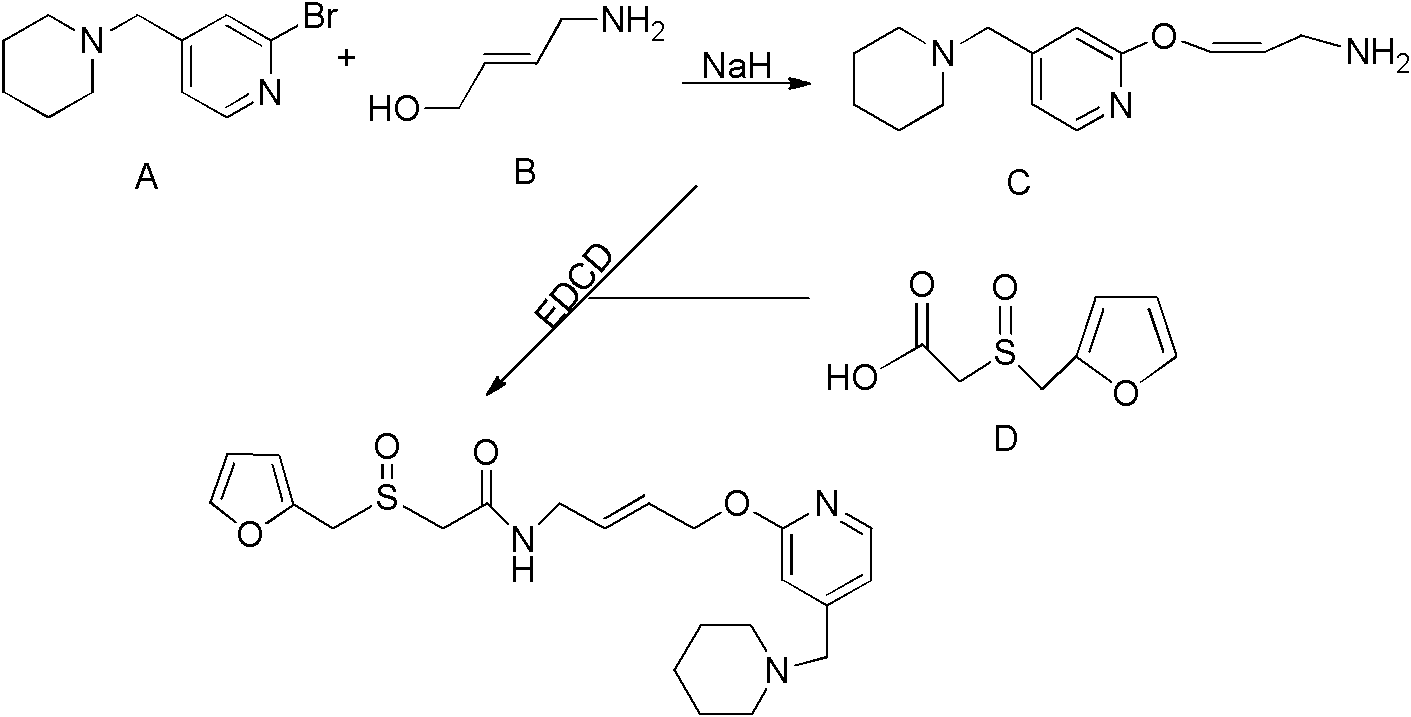
[0030]A. Preparation of 4-[4-(piperidin-1-yl-methylene)pyridin-2-yl-oxygen]-2(Z)-butene-1-amine Weigh 0.232g cis-4-chlorobutane Diene-1-amine hydrochloride was suspended in 10ml of dichloromethane, 0.5ml of triethylamine was added at room temperature, stirred until the solution was clear, and then 10ml was added to dissolve 2g of 4-(piperidin-1-yl-methylene) The dichloromethane solution of pyridin-2-ol is stirred at room temperature or under reflux until TLC shows that the reaction of compound 4-(piperidin-1-yl-methylene)pyridin-2-ol is complete, and the silica gel column chromatography is separated, and the chromatographic solution For methanol: ethyl acetate = 1:10, the compound 4-[4-(piperidin-1-yl-methylene)pyridin-2-yl-oxygen]-2(Z)-butene-1-amine 1.6g;
[0031] B. Preparation of 2-(2-furanmethylsulfoxide)acetic acid Dissolve 0.342g of 2-(furan-2-methyl)thioacetic acid in 10ml of ethanol, add 0.0004g of ammonium molybdate, stir for 10 minutes, at 25°C , add 0.7ml30%H2O2,...
Embodiment 2
[0034] A. Preparation of 4-[4-(piperidin-1-yl-methylene)pyridin-2-yl-oxygen]-2(Z)-butene-1-amine Weigh 0.28g cis-4-chlorobutyl Diene-1-amine hydrochloride was suspended in 9ml of dichloromethane, 0.5ml of triethylamine was added at room temperature, stirred until the solution was clear, and 2.5g of 4-(piperidin-1-yl-methylene ) dichloromethane solution of pyridin-2-ol, stirred at room temperature until TLC showed that the reaction of compound 4-(piperidin-1-yl-methylene) pyridin-2-ol was completed, and the silica gel column chromatography was separated, and the chromatographic solution was Methanol: ethyl acetate = 1:10, the compound 4-[4-(piperidin-1-yl-methylene)pyridin-2-yl-oxygen]-2(Z)-butene-1-amine 2.0 g;
[0035] B. Preparation of 2-(2-furanmethylsulfoxide) acetic acid Dissolve 0.32g of 2-(furan-2-methyl)thioacetic acid in 11ml of ethanol, add 0.0007g of ammonium molybdate, stir for 8 minutes, at 25°C , add 0.0005ml30%H2O2, heat preservation reaction for 12 minutes, a...
Embodiment 3
[0038] A. Preparation of 4-[4-(piperidin-1-yl-methylene)pyridin-2-yl-oxygen]-2(Z)-butene-1-amine Weigh 0.22g cis-4-chlorobutane Diene-1-amine hydrochloride was suspended in 11ml of dichloromethane, 0.6ml of triethylamine was added at room temperature, stirred until the solution was clear, and 9ml of 4-(piperidin-1-yl-methylene ) dichloromethane solution of pyridin-2-ol, room temperature or reflux stirring until TLC shows that compound 4-(piperidin-1-yl-methylene) pyridin-2-ol has reacted completely, separated by silica gel column chromatography, chromatographically The liquid is methanol: ethyl acetate = 1:10, and the compound 4-[4-(piperidin-1-yl-methylene)pyridin-2-yl-oxygen]-2(Z)-butene-1- Amine 2.5g;
[0039] B. Preparation of 2-(2-furanmethylsulfoxide) acetic acid Dissolve 0.38g of 2-(furan-2-methyl)thioacetic acid in 9ml of ethanol, add 0.0006g of ammonium molybdate, stir for 8 minutes, at 25°C , add 0.0007ml30%H2O2, heat preservation reaction 8 minutes, add 1ml satura...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com