Lafutidine tablet content detection method
A detection method, the lafutidine technology, is applied in the field of lafutidine tablet content detection, which can solve the problems of large material consumption, non-compliance with the separation requirements, cumbersome operating procedures, etc., and achieve less material consumption and accurate The effect of high degree and optimized chromatographic conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1. Preparation of sample solution
[0036] Prepare a lafutidine sample solution with a concentration of 0.1 mg / ml;
[0037] 2. Content detection
[0038] The detection conditions are:
[0039] The mobile phase is a mixed solution of acetonitrile / sodium pentanesulfonate-phosphate buffer solution with a volume ratio of 15:85;
[0040] The column temperature is 40°C;
[0041] The detection wavelength is 275nm.
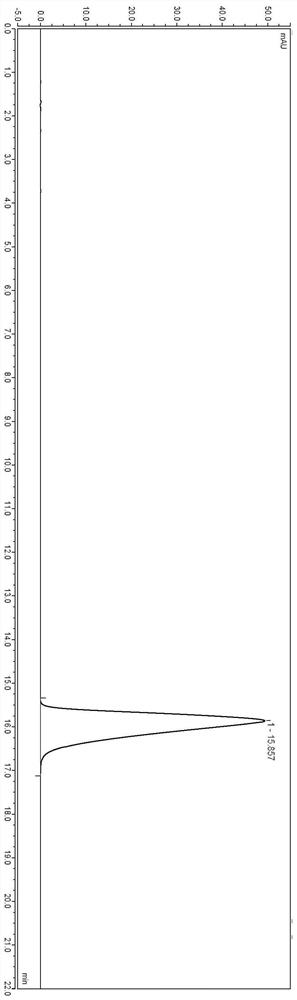
[0042] Under the above-mentioned chromatographic conditions, use high-performance liquid chromatography external standard method to detect the content of the sample solution, the injection volume is 25 μl, and the detection diagram is as follows figure 1 shown.
[0043] from figure 1 It can be seen that even if the sample concentration of lafutidine is reduced, the peak shape detected by high performance liquid chromatography is still good.
Embodiment 2
[0045] 1. Preparation of blank excipient solution
[0046] Take an appropriate amount of blank adjuvant according to the prescription ratio of lafutidine tablet, place it in a 100ml volumetric flask, add mobile phase to dissolve and dilute to the mark, shake up, and filter with a filter membrane to prepare a blank adjuvant solution.
[0047] 2. Prepare mixed solution
[0048] Take appropriate amount of lafutidine, lafutidine isomers and impurity reference substance, and configure it into a mixed solution.
[0049] 3. Content detection
[0050] Chromatographic conditions:
[0051] The mobile phase is a mixed solution of acetonitrile / sodium pentanesulfonate-phosphate buffer solution with a volume ratio of 15:85;
[0052] The column temperature is 40°C;
[0053] The detection wavelength is 275nm;
[0054] The chromatographic column is CAPCELL PAK C18 (150×4.6mm, 3μm);
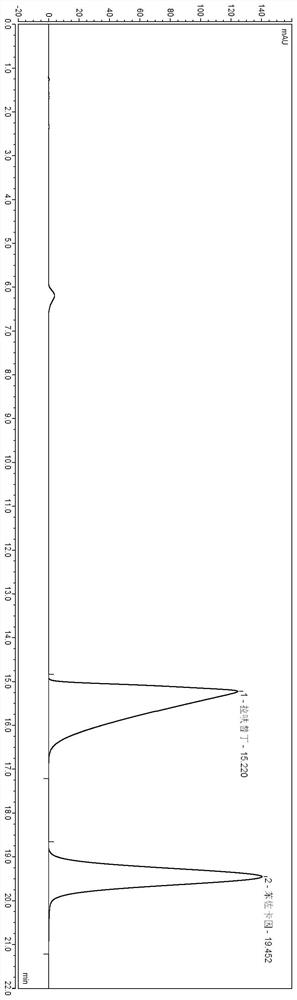
[0055] Adjust the flow rate so that the time for the main peak to emerge is about 15 minutes.
[0056] W...
Embodiment 3
[0061] 1. Prepare the test solution
[0062] Accurately weigh 20 tablets of the test product, grind them finely, accurately weigh an appropriate amount of fine powder, put the grinded fine powder equivalent to 10 mg of lafutidine in a 100ml measuring bottle, add an appropriate amount of mobile phase, and shake to make the lafutidine Futidine was dissolved and diluted to the mark, shaken up, filtered, and the filtrate was taken as the test solution, and 6 parts of the test solution were prepared in the same way.
[0063] 2. Prepare the reference solution
[0064] Get the appropriate amount of lafutidine reference substance, add mobile phase to dissolve and dilute the reference substance solution prepared.
[0065] 3. Content detection
[0066] Chromatographic conditions:
[0067] The mobile phase is a mixed solution of acetonitrile / sodium pentanesulfonate-phosphate buffer solution with a volume ratio of 15:85;
[0068] The column temperature is 40°C;
[0069] The detection...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com