Ranitidine hydrochloride lipidosome capsule and new application thereof
A technology of ranitidine hydrochloride fat and ranitidine hydrochloride, which is applied in the field of medicine, can solve the problems of high production cost of freeze-dried powder injection, high environmental requirements, and poor quality of finished products, so as to improve the drug therapeutic index and production process Simple, low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
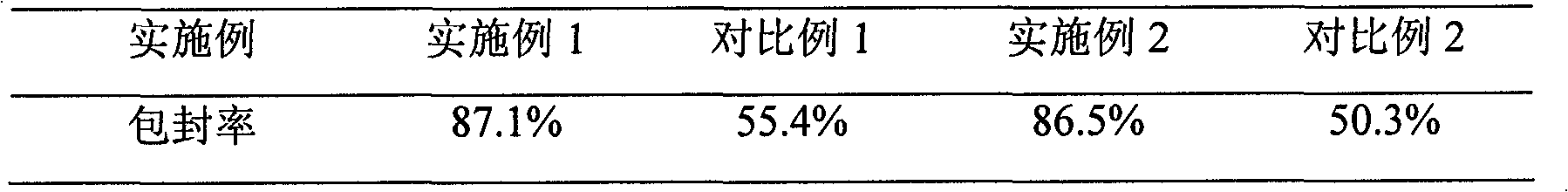
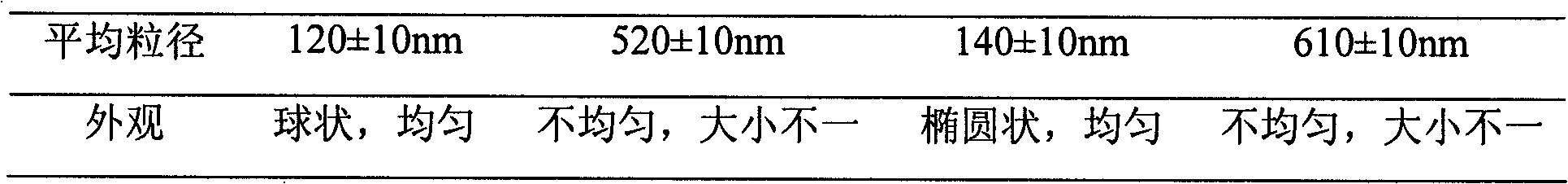
Examples
Embodiment 1
[0036] Example 1 Preparation of ranitidine hydrochloride liposome
[0037] Prescription: Ranitidine Hydrochloride 150g
[0038] Egg Yolk Lecithin 750g
[0039] Cholesterol 75g
[0040]Sodium Glycocholate 150g
[0041] Tween 80 150g
[0042] Preparation Process
[0043] (1) Dissolve 750g egg yolk lecithin, 75g cholesterol, and 150g sodium glycocholate in 3000ml ethanol, mix well, and remove ethanol under reduced pressure on a rotary thin film evaporator to obtain a phospholipid film;
[0044] (2) Add 1500ml of acetic acid-sodium acetate buffer solution with pH=4.0, stir to completely hydrate the phospholipid membrane, and then homogenize and emulsify at a high speed for 20min with a tissue masher at a speed of 12000r / min to prepare a blank liposome suspension;
[0045] (3) 150g of ranitidine hydrochloride and 150g of Tween 80 were dissolved in 3000ml of water, added to the blank liposome suspension, kept at 65°C, and homogeneously emulsified for 10min ...
Embodiment 2
[0054] Example 2 Preparation of ranitidine hydrochloride liposome
[0055] Prescription: Ranitidine Hydrochloride 75g
[0056] Yolk Lecithin 675g
[0057] Cholesterol 375g
[0058] Sodium Glycocholate 450g
[0059] Tween 80 300g
[0060] Preparation Process
[0061] (1) 675g egg yolk lecithin, 375g cholesterol, and 450g sodium glycocholate were dissolved in 7500ml ethanol, mixed uniformly, and ethanol was removed under reduced pressure on a rotary thin film evaporator to obtain a phospholipid film;
[0062] (2) Add pH = 5.0 sodium dihydrogen phosphate-disodium hydrogen phosphate buffer solution 2500ml, stir to completely hydrate the phospholipid membrane, then homogenize and emulsify for 10 minutes with a tissue masher at a speed of 15000r / min to prepare a blank lipid body suspension;
[0063] (3) 75g of ranitidine hydrochloride and 300g of Tween 80 were dissolved in 4000ml of water, added to the blank liposome suspension, kept at 55°C, and homogeneously emulsified f...
Embodiment 3
[0074] Example 3 Preparation of Ranitidine Hydrochloride Capsules
[0075] Ranitidine hydrochloride liposome (calculated as ranitidine) 75g
[0076] Microcrystalline Cellulose 75g
[0077] Lactose 50g
[0078] Povidone K30 5g
[0079] Talc powder 5g
[0080] Preparation Process
[0081] (1) pulverize the liposome containing 75g ranitidine hydrochloride, cross 80 mesh sieves, and set aside;
[0082] (2) Take by weighing 75g microcrystalline cellulose, 50g lactose, cross 80 mesh sieves, mix, set aside;
[0083] (3) Mix the above raw and auxiliary materials evenly, add 5% povidone K 30 100ml of 80% ethanol solution is used to make soft materials, pass through a 20-mesh sieve to granulate, dry at 60°C, and granulate through a 18-mesh sieve to obtain ranitidine hydrochloride granules;
[0084] (4) Fill the dried granules into capsules to prepare ranitidine hydrochloride capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com