Preparation method of ranitidine hydrochloride
A technology of ranitidine hydrochloride and ranitidine base, which is applied in the field of medicine, can solve the problems of inapplicability to mass production, high processing costs, and cumbersome steps, and achieve the effects of reducing production costs, simple steps, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The preparation method of described ranitidine hydrochloride comprises the following processing steps:
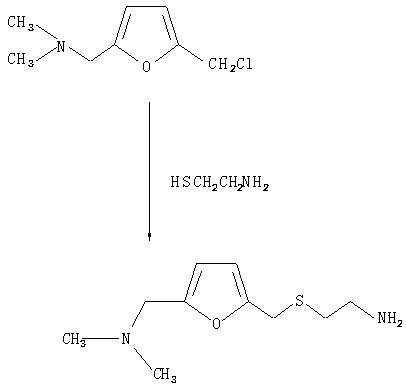
[0026] (1) Add 0.3 mol of 2-chloromethyl-5-dimethylaminofuran and 0.3 mol of cysteamine to basic alumina, reflux and stir for 20 minutes, cool to room temperature, add haloalkane, and reflux for 1 to 2.5 hours , complete S-alkylation to obtain 2-[[[5-[(dimethylamino)methyl]furanmethyl]thio]ethylamine;
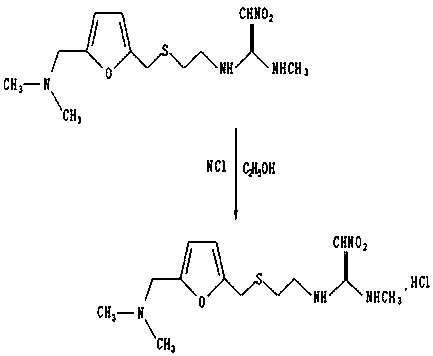
[0027] (2) Put 0.1 mol of 2-[[[5-[(dimethylamino)methyl]furanmethyl]thio]ethylamine prepared in step (1) into water, add 0.05 mol of N-methyl-1-methylthio-2-nitroethyleneamine, heated and stirred for 3 to 4 hours, then added hydrochloric acid to make it acidified to obtain a mixed reactant, which was added to chloroform to extract Finally, add potassium carbonate to alkalize, then add isopropanol to azeotropically remove water, then add n-ethane to cool down and crystallize to obtain ranitidine base;
[0028] (3) Use the ranitidine base prepared in step (2) to track...
Embodiment 2
[0037] The preparation method of described ranitidine hydrochloride comprises the following processing steps:
[0038] (1) Add 0.3 mol of 2-chloromethyl-5-dimethylaminofuran and 0.5 mol of cysteamine to basic alumina, reflux and stir for 20 minutes, cool to room temperature, add haloalkane, and reflux for 1 to 2.5 hours. Complete S-alkylation to produce 2-[[[5-[(dimethylamino)methyl]furylmethyl]thio]ethylamine;
[0039] (2) Put 0.1 mol of 2-[[[5-[(dimethylamino)methyl]furanmethyl]thio]ethylamine prepared in step (1) into water, and add 0.1 molN-methyl-1-methylthio-2-nitroethyleneamine, heated and stirred for 3 to 4 hours, then added hydrochloric acid to make it acidified to obtain a mixed reactant, which was added to chloroform, and after extraction, Add potassium carbonate to alkalinize, then add isopropanol to remove water azeotropically, then add n-ethane to cool down and crystallize to obtain ranitidine base;
[0040] (3) Use the ranitidine base prepared in step (2) to t...
Embodiment 3
[0045] The preparation method of described ranitidine hydrochloride comprises the following processing steps:
[0046] (1) Add 0.3 mol of 2-chloromethyl-5-dimethylaminofuran and 0.6 mol of cysteamine to basic alumina, reflux and stir for 20 minutes, cool to room temperature, add halogenated alkanes, and reflux for 1 to 2.5 hours to complete S-alkylation to produce 2-[[[5-[(dimethylamino)methyl]furylmethyl]thio]ethylamine;
[0047] (2) Put 0.1 mol of 2-[[[5-[(dimethylamino)methyl]furanmethyl]thio]ethylamine prepared in step (1) into water, and add 0.2 mol of N-methyl-1-methylthio-2-nitroethyleneamine, heated and stirred for 3 to 4 hours, then added hydrochloric acid to make it acidified to obtain a mixed reactant, which was added to chloroform to extract Finally, add potassium carbonate to alkalize, then add isopropanol to azeotropically remove water, then add n-ethane to cool down and crystallize to obtain ranitidine base;
[0048] (3) Use the ranitidine base prepared in ste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com