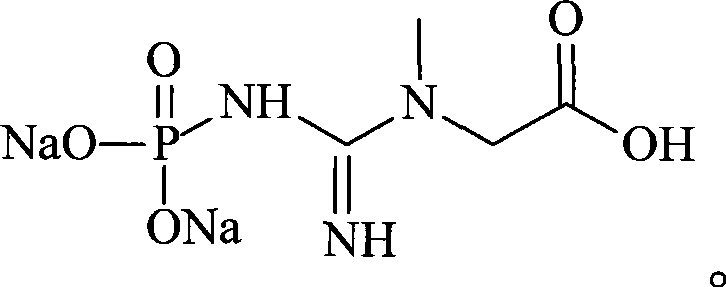
Creatine phosphate sodium lipidosome freeze-dried preparation and preparation method thereof
A technology of creatine phosphate sodium and liposome freeze-drying, which can be used in liposome delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve the problem of liposome particle size increase and poor hydration resolubility , liposome leakage and other problems, to achieve the effect of improving stability, product quality and reducing drug side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
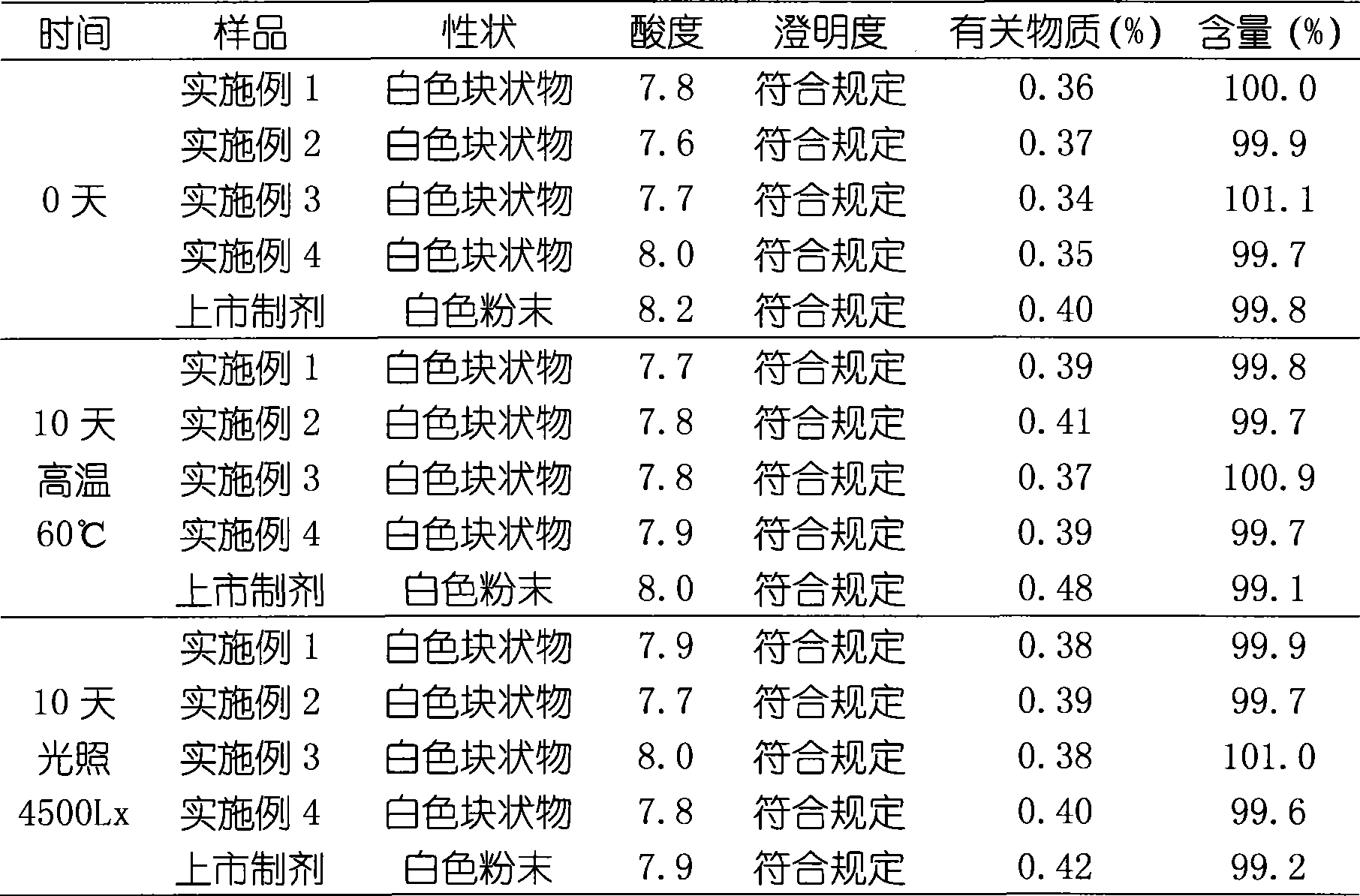
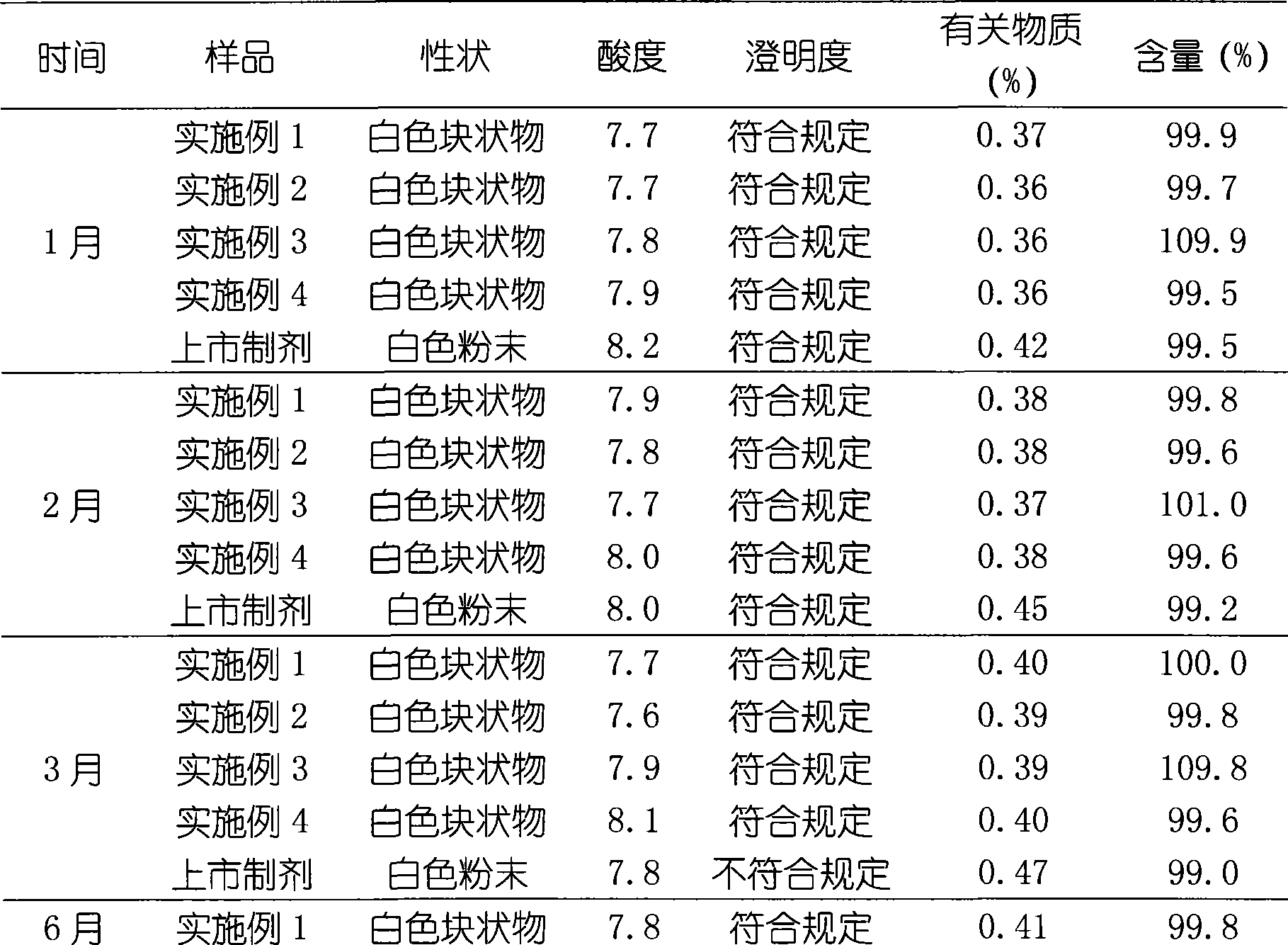
Embodiment 1
[0031] Embodiment 1 Preparation of creatine phosphate sodium liposome freeze-dried preparation
[0032] Prescription (100 bottles): Sodium Creatine Phosphate 50g
[0033] Soy Lecithin 75g
[0034] Cholesterol 20g
[0035] Vitamin E 10g
[0036] Mannitol 40g
[0037] Preparation Process
[0038](1) Weigh 75g of soybean lecithin, 20g of cholesterol and 10g of vitamin E and dissolve them in 800ml of acetone, heat and melt, add a pH value of 7.8 phosphate buffer solution, heat to evaporate the acetone, transfer to a tissue masher, and stir at a high speed for 10 minutes, filter, sterilize, and sonicate for 10 minutes;
[0039] (2) In a sterile room, under 100-grade conditions, add 50 g of sterilized creatine phosphate sodium and 40 g of mannitol and dissolve, filter, fill in a vial, and freeze-dry to obtain creatine phosphate sodium liposome jelly Dry powder injection.
Embodiment 2
[0040] Embodiment 2 Preparation of creatine phosphate sodium liposome freeze-dried preparation
[0041] Prescription (100 bottles): Sodium Creatine Phosphate 100g
[0043] Cholesterol 25g
[0044] Sodium bisulfite 37.5g
[0045] Glycine 100g
[0046] Mannitol 100g
[0047] Preparation Process
[0048] (1) Weigh 150g of egg yolk lecithin and 25g of cholesterol, dissolve it in 1000ml of a mixture of tert-butanol and isopropanol with a volume ratio of 1:1, heat and melt, add a phosphate buffer solution with a pH value of 7.8, heat and distill off tert-butanol and isopropanol Alcohol, transferred to a tissue masher, stirred at high speed for 30 minutes, filtered, sterilized, and ultrasonicated for 20 minutes;
[0049] (2) Add 100 g of sterilized creatine phosphate sodium, 37.5 g of sodium bisulfite, 100 g of lyophilized support agent glycine and 100 g of mannitol in a sterile roo...
Embodiment 3
[0050] Example 3 Preparation of Creatine Phosphate Sodium Liposome Lyophilized Preparation
[0051] Prescription (100 bottles): Sodium Creatine Phosphate 50g
[0052] Hydrogenated egg yolk lecithin 25g
[0053] Ascorbyl Palmitate 37.5g
[0054] Glycine 31g
[0055] Mannitol 31g
[0056] Preparation Process
[0057] (1) Weigh 25g of hydrogenated egg yolk lecithin and 37.5g of ascorbyl palmitate, dissolve them in 500ml of a mixture of tert-butanol and isopropanol with a volume ratio of 1:1, heat and melt, add a pH value of 7.8 phosphate buffer solution, heat and evaporate tert-butanol and isopropanol, transferred to a tissue masher, stirred at high speed for 20 minutes, filtered, sterilized, and ultrasonicated for 10 minutes;
[0058] (2) In a sterile room, under the condition of grade 100, add 50 g of sterilized creatine phosphate sodium and 31 g of freeze-dried support agents glycine and 31 g of mannitol and dissolve the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com