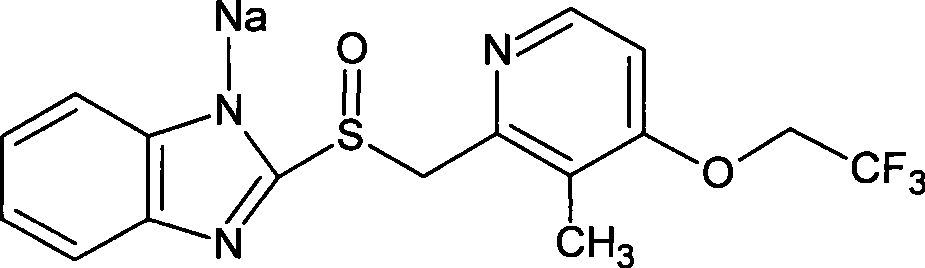
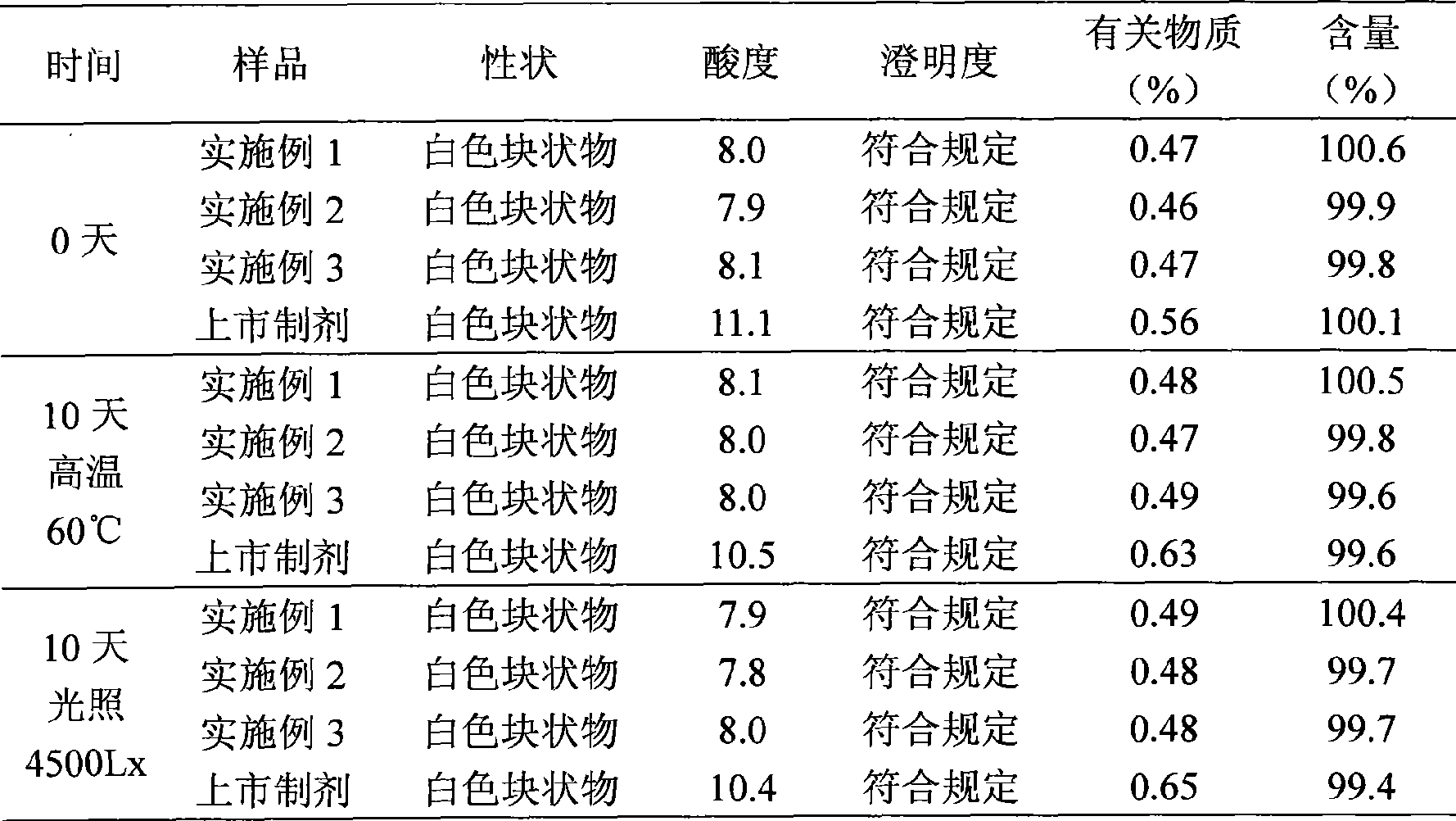
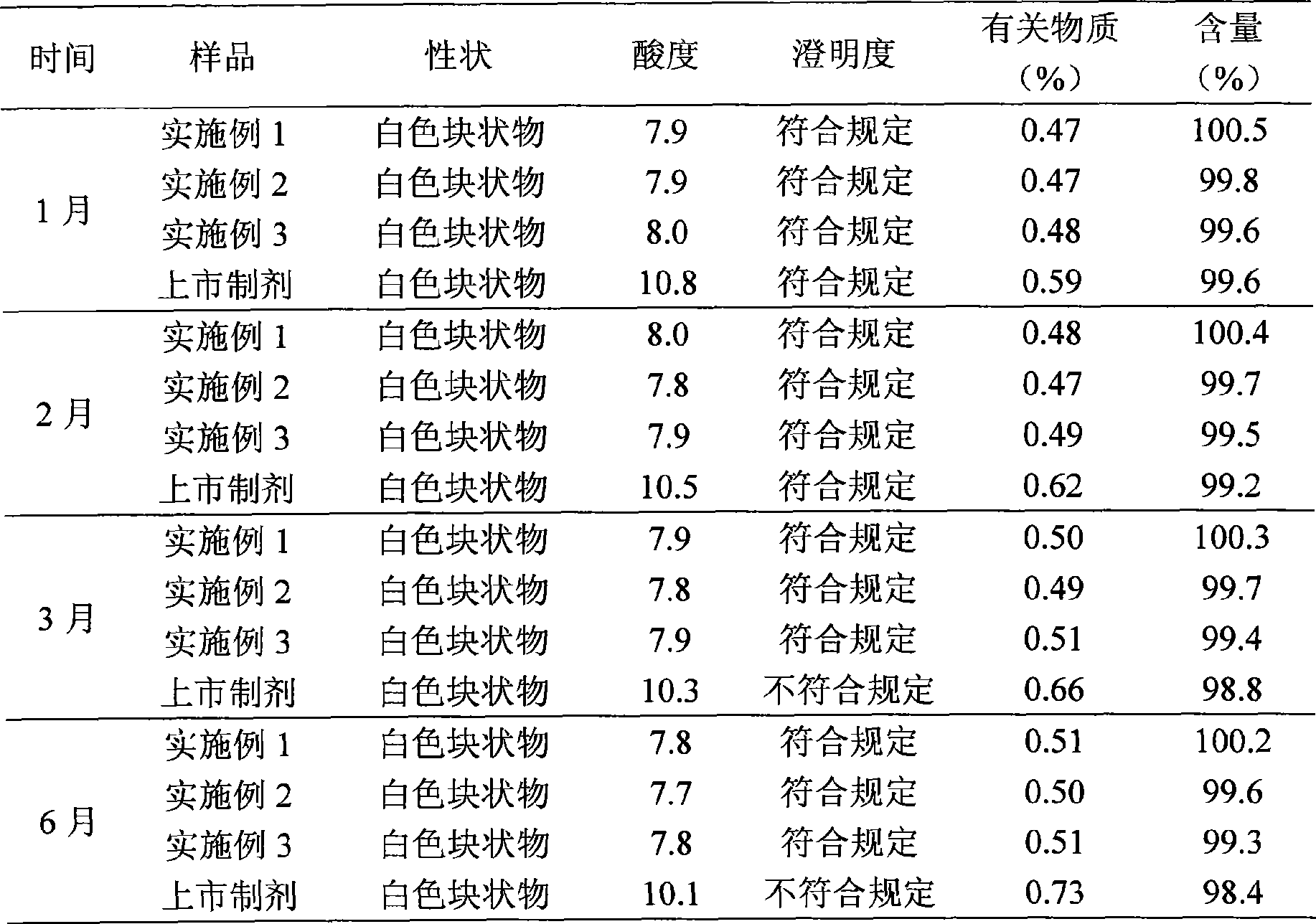
Lansoprazole sodium submicron emulsion freeze-drying preparation
A technology of lansoprazole sodium and freeze-dried preparations, which is applied in the field of submicron emulsion freeze-dried preparations of lansoprazole sodium and its preparation, and can solve problems such as difficult industrial scale-up production, easy destruction, and reduced production reproducibility , to achieve the effect of reducing drug side effects, ensuring product quality, and product quality stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of Lansoprazole Sodium Submicroemulsion Freeze-dried Preparation
[0050] Formula: Lansoprazole 15g
[0052] Polyacrylate 75g
[0053] Poloxamer 188 100g
[0054] Trehalose 120g
[0055] Povidone K15 30g
[0056] Preparation Process
[0057] (1) 15g lansoprazole and 2g sodium hydroxide are dissolved in 500ml water, 75g polyacrylate is dissolved in 200ml acetone, the two are mixed and stirred for 15min, and the rotating speed is 800r / min to make W / O type emulsion;
[0058] (2) Dissolve 120g trehalose and 30g povidone K15 in 1000ml water, add the solution to the above-mentioned W / O emulsion, stir at room temperature for 30min, and rotate at 200r / min to make a W / O / W double emulsion;
[0059] (3) Add the above-mentioned W / O / W type double emulsion into the aqueous solution of 100g poloxamer 188, stir at room temperature for 60min, rotate at a speed of 500r / min, evaporate acetone under redu...
Embodiment 2
[0091] The preparation of embodiment 2 lansoprazole sodium submicron emulsion preparation
[0092] Formula: Lansoprazole 30g
[0094] Polylactic acid 180g
[0095] Tween 80 60g
[0096] Glucose 180g
[0097] Povidone K30 18g
[0098] Preparation Process
[0099] (1) Dissolve 30g of lansoprazole and 3.5g of sodium hydroxide in 1000ml of water, dissolve 180g of polylactic acid in 500ml of isopropanol, mix and stir for 30min at a speed of 200r / min to make a W / O emulsion ;
[0100] (2) Dissolve 18g of povidone K30 and 180g of glucose in 500ml of water, add the solution to the above-mentioned W / O emulsion, stir at room temperature for 40min, and rotate at 800r / min to make a W / O / W type double emulsion;
[0101] (3) Add the above-mentioned W / O / W type double emulsion into the aqueous solution of 60g Tween 80, stir at room temperature for 30min, rotate at a speed of 200r / min, evaporate isopropanol under reduced pressur...
Embodiment 3
[0104] The preparation of embodiment 3 lansoprazole sodium submicron emulsion preparation
[0105] Formula: Lansoprazole 30g
[0106] Sodium hydroxide 3.8g
[0107] Polylactic / glycolic acid copolymer 150g
[0108] Lecithin 90g
[0109] Maltose 100g
[0110] Dextran 40 40g
[0111] Preparation Process
[0112] (1) 30g lansoprazole and 3.8g sodium hydroxide were dissolved in 1000ml water, 150g polylactic acid / glycolic acid copolymer was dissolved in 800ml dichloromethane, the two were mixed and stirred for 20min, and the rotating speed was 500r / min to prepare W / O type emulsion;
[0113] (2) Dissolve 40g of dextran 40 and 100g of maltose in 500ml of water, add the solution to the above-mentioned W / O type emulsion, stir at room temperature for 35min, and rotate at 400r / min to make W / O / W type double emulsion;
[0114] (3) Add the above-mentioned W / O / W type double emulsion into the aqueous solution of 90g lecithin, stir at room temperature for 40...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com