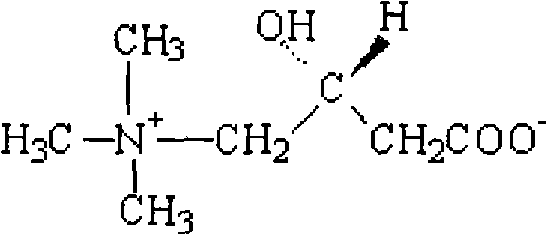
Levocarnitine liposomes injection
A levocarnitine lipid and injection technology, applied in the field of medicine, can solve the problems of easy deterioration and poor resolubility of powder injections, and achieve the effects of improved stability, stable product quality, and excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
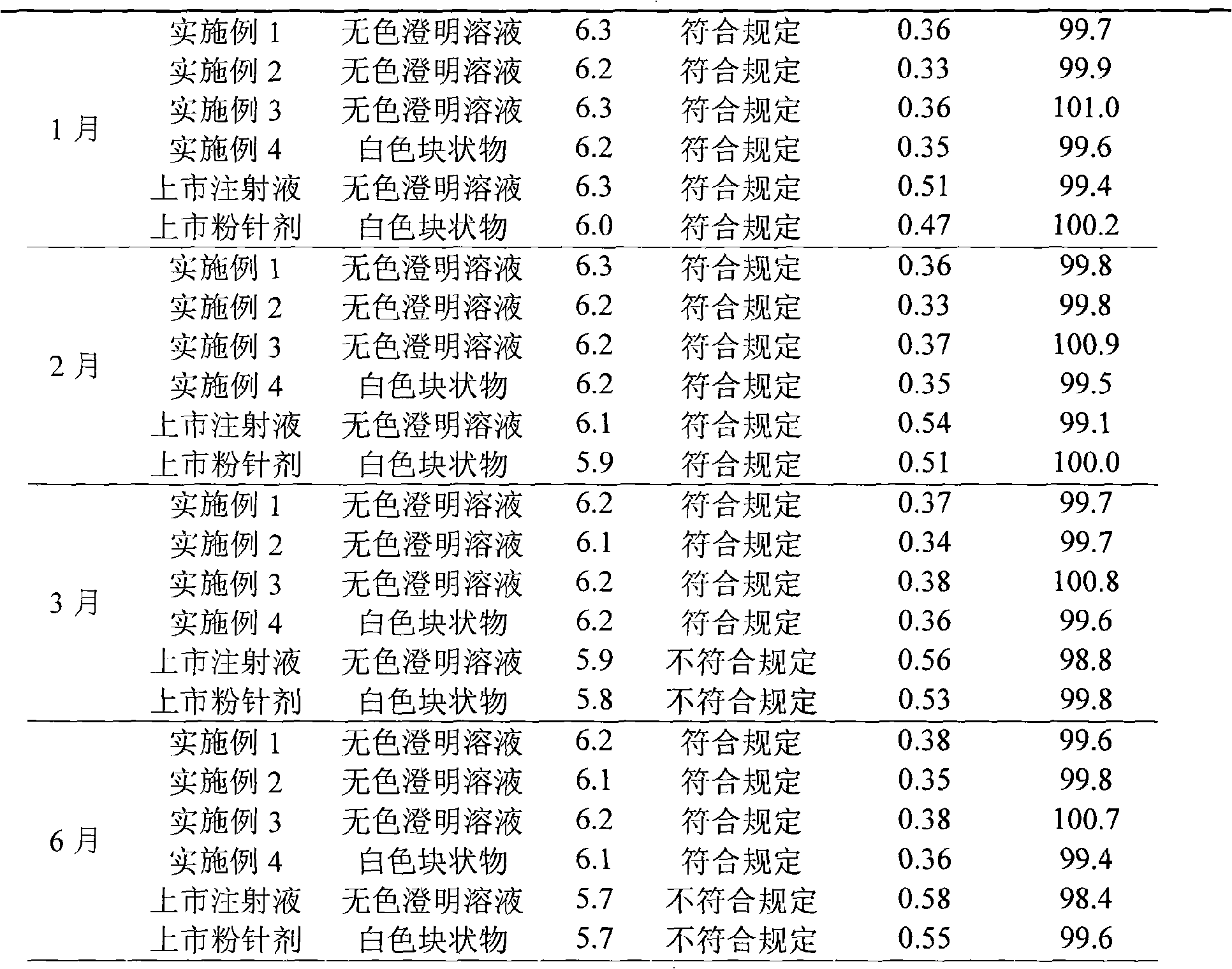
[0048] Example 1 Preparation of Levocarnitine Liposome Injection
[0049] Prescription (100 bottles): Levocarnitine 25g
[0050] Soy Lecithin 180g
[0051] Cholesterol 45g
[0052] Vitamin E 6g
[0053] Preparation Process
[0054] (1) Dissolve 180g of soybean lecithin, 45g of cholesterol and 6g of vitamin E in 1500ml of isopropanol, slowly inject 1500ml of 0.1mol / L ammonium sulfate solution under stirring, heat and stir to remove the isopropanol, and put it in an ice bath for 10 minutes of ultrasound , to obtain blank liposomes;
[0055] (2) Blank liposome is placed in dialysis bag, seals, and dialysis bag is placed in 0.9% sodium chloride solution 3000ml and dialyzes 20 hours, removes the ammonium sulfate in liposome external phase;
[0056] (3) Dissolve 25g of levocarnitine in 800ml of water, keep the dialyzed blank liposome at 60°C, slowly add the aqueous solution of levocarnitine under stirring, continue to keep warm for 20min, add 1000ml of 0.9% sodium chloride s...
Embodiment 2
[0058] Example 2 Preparation of Levocarnitine Liposome Injection
[0059] Prescription (100 bottles): Levocarnitine 50g
[0060] Soy Lecithin 250g
[0061] Cholesterol 40g
[0062] tert-Butyl p-Hydroxyanisole 5g
[0063] Preparation Process
[0064] (1) Dissolve 250g of soybean lecithin, 40g of cholesterol and 5g of tert-butyl p-hydroxyanisole in 2000ml of tert-butanol, slowly inject 2000ml of 0.05mol / L ammonium sulfate solution under stirring, heat and stir to remove tert-butanol, place Sonicate in an ice bath for 20 minutes to obtain blank liposomes;
[0065] (2) blank liposome is placed in the dialysis bag, seals, and the dialysis bag is placed in 4000ml of 10% glycerol solution and dialyzed for 24 hours, removes the ammonium sulfate in the liposome external phase;
[0066] (3) 50g levocarnitine is dissolved in 2000ml water, and the blank liposome that dialyzes is incubated at 60 ℃, slowly adds the aqueous solution of levocarnitine under stirring, continues to keep...
Embodiment 3
[0068] Example 3 Preparation of Levocarnitine Liposome Injection
[0069] Prescription (100 bottles): L-Carnitine 100g
[0070] Soy Lecithin 900g
[0071] Cholesterol 600g
[0072] Vitamin E 36g
[0073] Preparation Process
[0074] (1) Dissolve 900g of soybean lecithin, 600g of cholesterol and 36g of vitamin E in 10,000ml of ethanol, slowly inject 10,000ml of 0.03mol / L ammonium sulfate solution under stirring, heat and stir to remove ethanol, put it in an ice bath and ultrasonically for 20min, and obtain blank fat plastid;
[0075] (2) Blank liposome is placed in dialysis bag, seals, and dialysis bag is placed in 5% glucose solution 15000ml and dialyzes 26 hours, removes the ammonium sulfate in liposome external phase;
[0076] (3) Dissolve 100g of levocarnitine in 5000ml of water, heat the dialyzed blank liposome at 60°C, slowly add the aqueous solution of levocarnitine under stirring, continue to keep warm for 20min, add 1000ml of 5% glucose solution and pH Value ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com