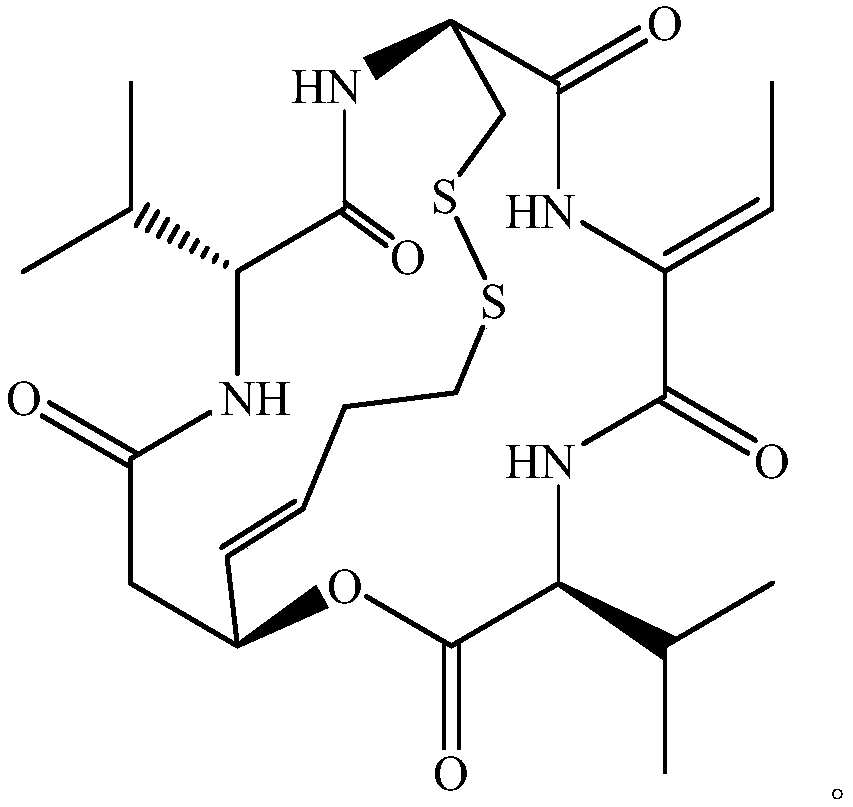
A kind of method for preparing amorphous romidepsin
A romidepsin and amorphous technology, applied in the field of medicine, can solve the problems of unstable process, unsuitable for industrial production, difficult removal of dioxane, etc., and achieves the effects of stable freeze-drying process and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: Solubility experiment of romidepsin in ethanol aqueous solution with different concentrations
[0034] Take 6 parts of 20mg romidepsin and put them into 10ml ethanol aqueous solution with different volume percentage respectively, stir at room temperature for 60 minutes, then take samples respectively and use 0.45μm membrane (SCAA-103, Shanghai Anpu Experiment Technology Co., Ltd. ) to filter, the sample obtained uses HPLC external standard method to analyze its content and calculate its solubility. See Table 1 for the solubility of romidepsin in various concentrations of ethanol at room temperature.
[0035] Table 1: Solubility of romidepsin in aqueous ethanol solutions of different concentrations
[0036] Ethanol concentration (%)
Embodiment 2
[0037] Embodiment 2: The influence of the pre-cooling temperature of the freeze-drying chamber of the freeze-dryer on the form of romidepsin
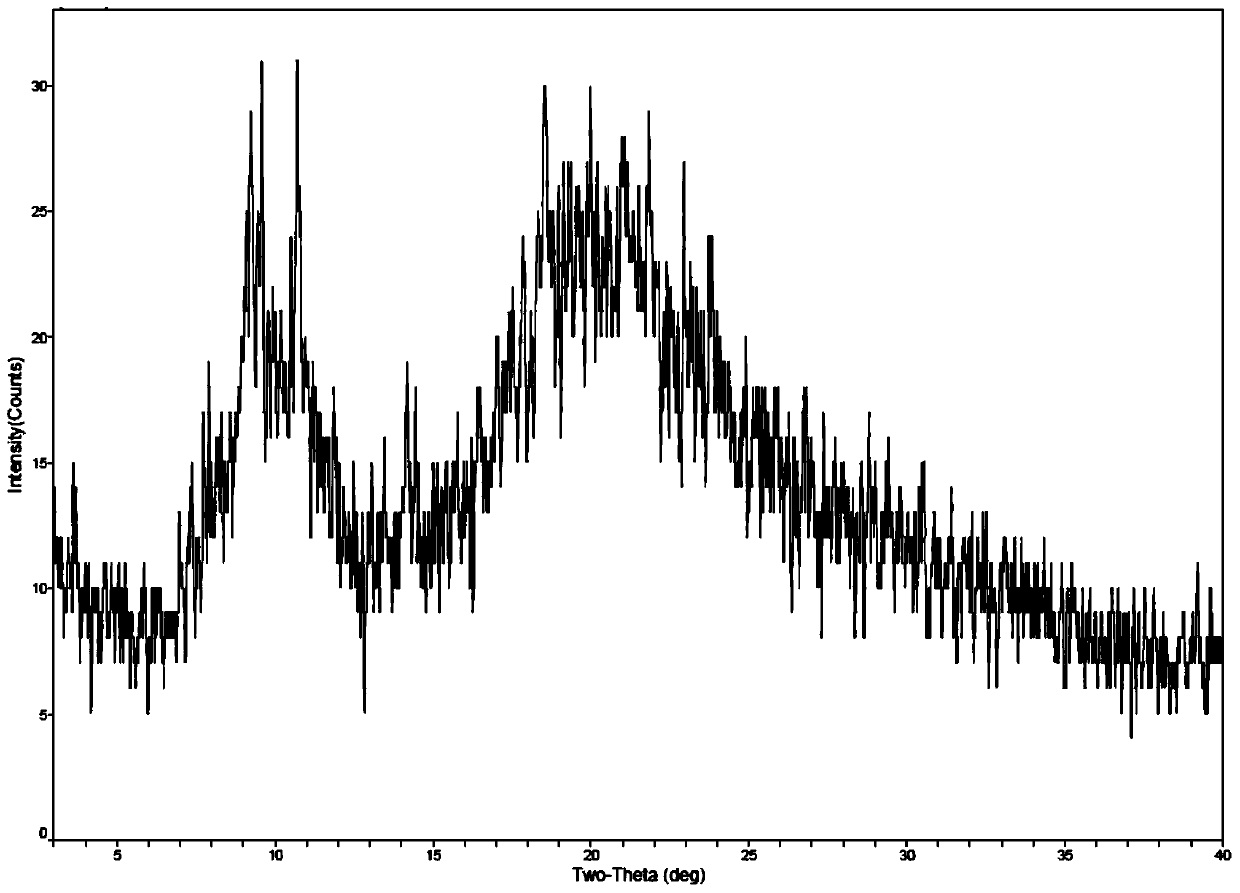
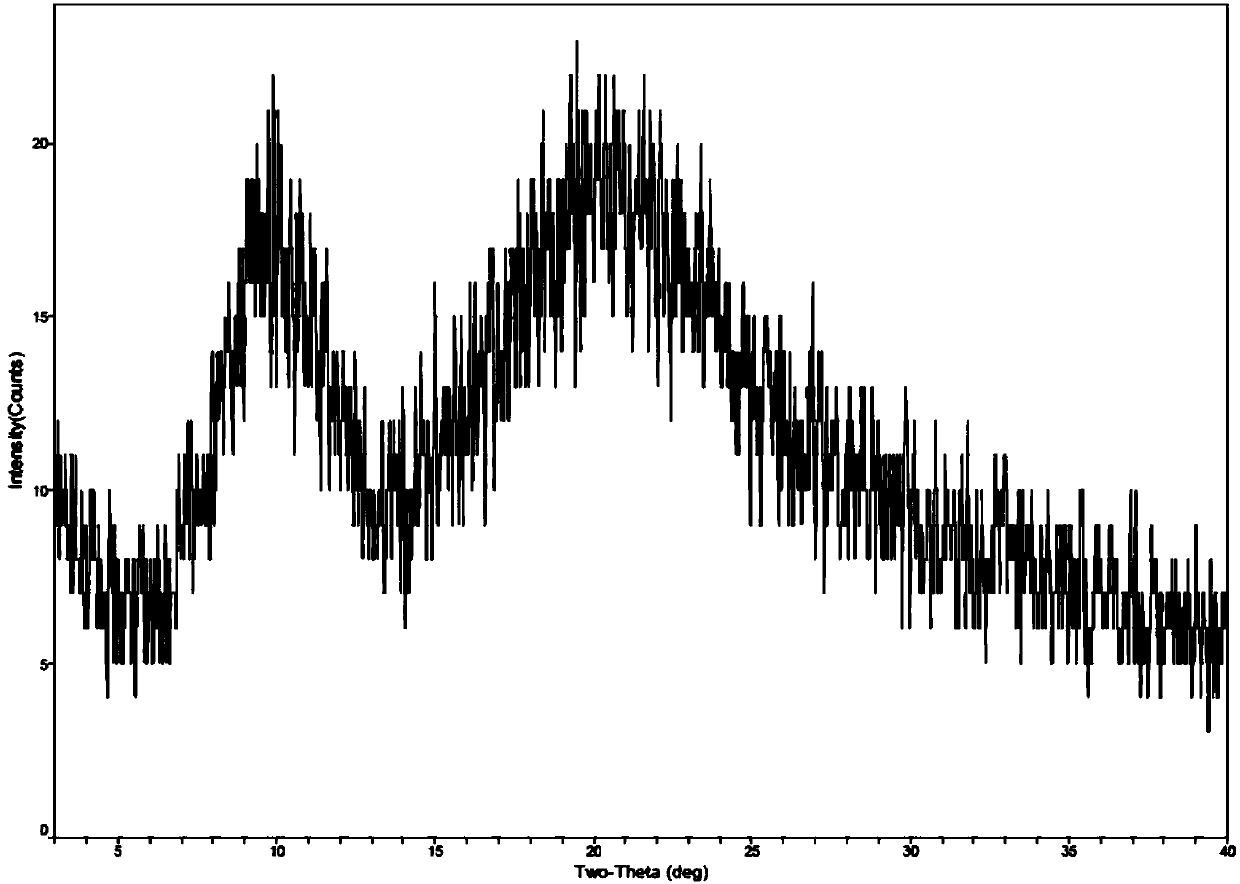
[0038]Take 1g of romidepsin and dissolve in 50mL of ethanol, then take 9mL of this solution and add 21mL of water to mix well. Then place the solution in the freeze-drying chamber at room temperature, pre-cooled to -5 ° C, -20 ° C and -70 ° C in the freeze dryer for pre-freezing (-40 ° C for 4 hours), through drying (-20 ℃ for 16 hours; -10°C for 6 hours; 30°C for 8 hours; 40°C for 8 hours) to obtain romidepsin as a solid. The solid form of romidepsin was determined by X-ray powder diffractometer, and the results are shown in Table 2. See figure 1 See figure 2 .
[0039] Table 2: Effect of pre-cooling of freeze-drying chamber temperature on freeze-dried romidepsin
[0040]
Embodiment 3
[0041] Example 3: Effects of different raw material concentrations and different ethanol concentrations on freeze-dried romidepsin
[0042] Take 0.3, 0.5, 0.75, 1.0 and 1.25g romidepsin and dissolve them in 50mL ethanol respectively, then take appropriate above-mentioned solutions and add water to make sample solutions with ethanol concentration as shown in Table 3, mix well and place in Pre-cooled to -20°C in a freeze-drying bin (-40°C for 4 hours), through drying (-20°C for 16 hours; -10°C for 6 hours; 30°C for 8 hours; 40°C for 8 hours) ) to obtain romidepsin solid. The solid state of romidepsin was determined by X-ray powder diffractometer, and the results are shown in Table 3.
[0043] Table 3: Effects of different raw material concentrations and different ethanol concentrations on freeze-dried romidepsin
[0044]
[0045] Note: " / " means no experiments are scheduled
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com