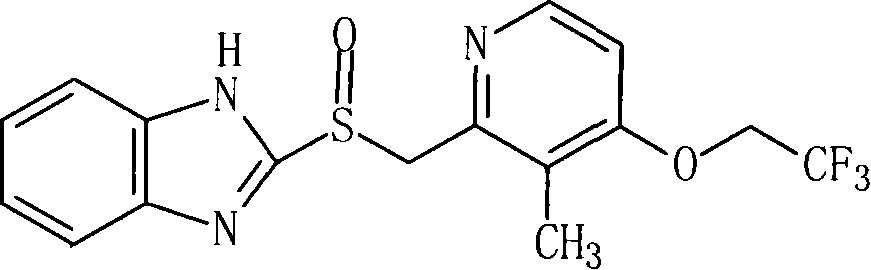
Lansoprazole freeze-dried injection
A technology for freeze-dried powder injection and lansoprazole, which is applied in the field of lansoprazole freeze-dried powder for injection, can solve the problems of low bioavailability, slow oral absorption, inability to sterilize at high temperature, etc., and achieves stable storage and transportation process. , The effect of low content of related substances and high level of sterility assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
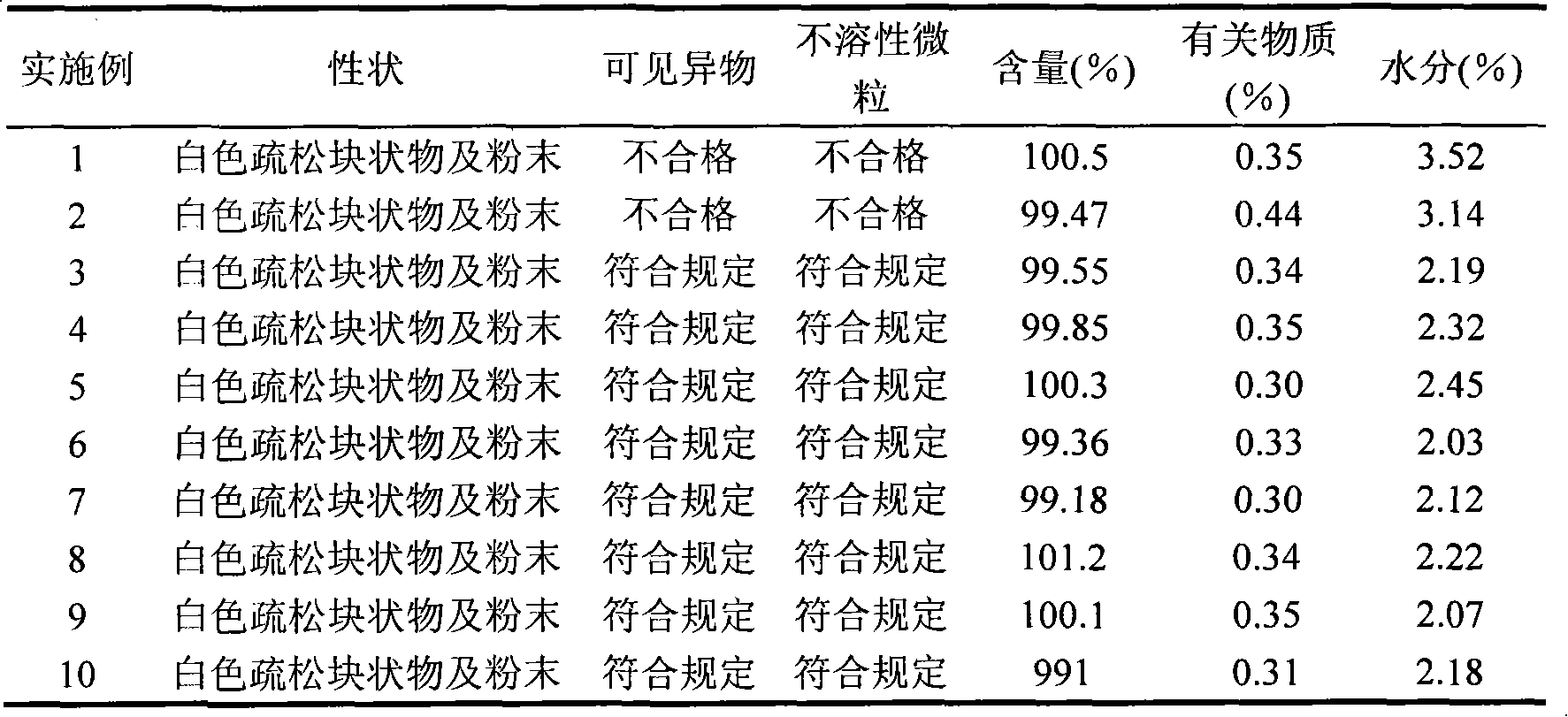
Examples
Embodiment 1
[0022] Ordinary method prepares lansoprazole freeze-dried powder injection (30mg specification)
[0023] prescription
[0024] Lansoprazole 30g
[0025] Mannitol 50g
[0026] Add water for injection to 3000ml
[0027] A total of 1000 bottles were made
[0028] Get prescription quantity lansoprazole and put in the container, add the water for injection of 80% amount of total volume, add alkali and stir to make it dissolve and mix uniformly, then add prescription quantity mannitol, stir to make it dissolve and mix uniformly, measure the lansoprazole in the solution After the content of soprazole, add water for injection to dilute to the full amount, under sterile conditions, filter with a 0.22 μm microporous membrane until it is clear, fill the filtrate in a sterile vial, and partially plug it with a butyl rubber stopper. Pack into trays, send them into the freeze dryer, close the door, turn on the freeze dryer, use heat transfer oil to cool the plate layer, so that the prod...
Embodiment 2
[0030] Ordinary method prepares lansoprazole freeze-dried powder injection (15mg specification)
[0031] prescription
[0032] Lansoprazole 15g
[0033] Mannitol 25g
[0034] Add water for injection to 1500ml
[0035] A total of 1000 bottles were made
[0036] Get prescription quantity lansoprazole and put in the container, add the water for injection of 80% amount of total volume, add alkali and stir to make it dissolve and mix uniformly, then add prescription quantity mannitol, stir to make it dissolve and mix uniformly, measure the lansoprazole in the solution After the content of soprazole, add water for injection to dilute to the full amount, under sterile conditions, filter with a 0.22 μm microporous membrane until it is clear, fill the filtrate in a sterile vial, and partially plug it with a butyl rubber stopper. Load the plate, send it to the freeze dryer, close the door, turn on the freeze dryer, use heat transfer oil to cool the plate layer, and reduce the produc...
Embodiment 3
[0038] prescription
[0039] Lansoprazole 30g
[0040] Mannitol 50g
[0041] Add water for injection to 3000ml
[0042] A total of 1000 bottles were made
[0043] Get lansoprazole and put in the container, add total volume 30% water for injection, add alkali and stir to make it dissolve and mix uniformly to make lansoprazole concentrated solution, then add mannitol, stir to make it dissolve and mix uniformly, add Acid counter-adjusting the pH value of the solution to 10.3, leaving it to stand to make the solution crystallize, filtering out the precipitated crystals with a microporous filter membrane, measuring the content of lansoprazole in the solution, adjusting the pH value of the filtrate to 11.5 according to the measurement results and constant volume with water for injection To the full amount, under sterile conditions, filter with a 0.22 μm microporous membrane until it becomes clear, fill the filtrate in a sterile vial, partially plug it with a butyl rubber stopper, p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com