Romidepsin fat microsphere preparation and preparing method thereof
A technology of romidepsin and lipid microspheres, which is applied in the directions of emulsion delivery, freeze-drying delivery, tetrapeptide components, etc., can solve problems such as no reports of romidepsin lipid microsphere preparations, and achieve improved drug safety, Effect of improving compliance and improving targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
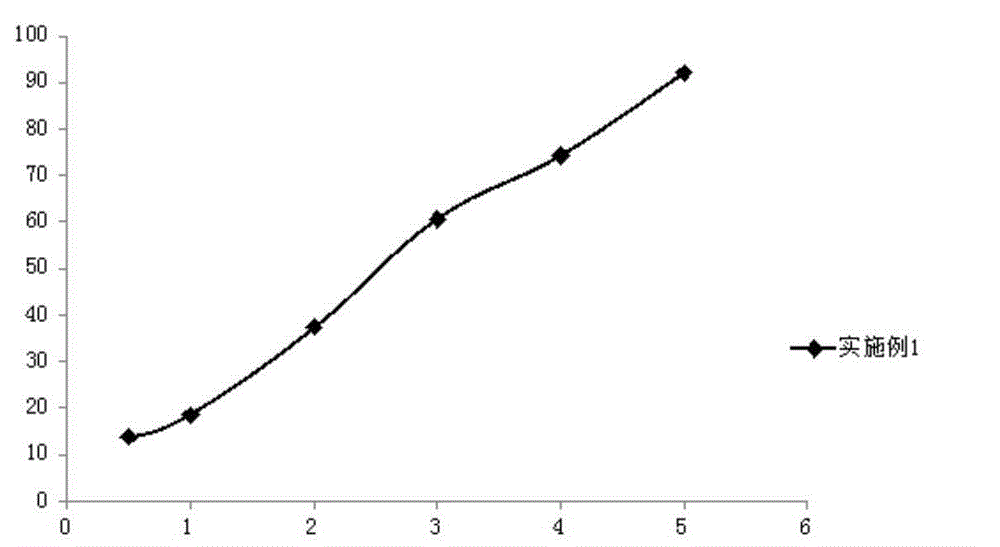
Embodiment 1
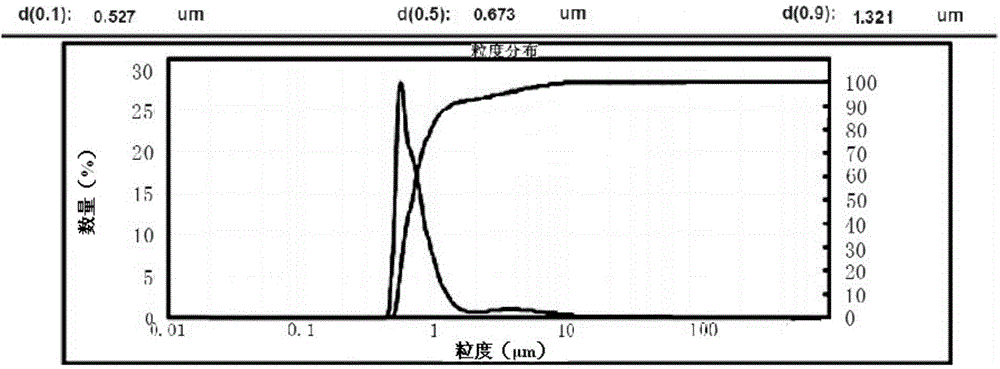
[0050] (1) Weigh 1.0g romidepsin, 5.0g soybean oil, 5.0g medium-chain fatty acid triglycerides, 1.5g soybean lecithin, 0.4g propylene glycol and 0.05g ethanol in a beaker, and use a high-speed shearing machine Shear at high speed for 3 minutes, heat and stir in a magnetic stirring water bath at 60°C at a stirring speed of 500rpm to completely dissolve and obtain an oil phase;
[0051] (2) Weigh 0.9g of sodium chloride and place it in a beaker, add 86.2g of water for injection, heat and stir in a magnetic stirring water bath at 60°C, and stir at a stirring speed of 500rpm to completely dissolve it to obtain an aqueous phase;
[0052] (3) under high-speed stirring, the oil phase and the water phase are mixed, and the pH value is adjusted to 7.0 to obtain colostrum;
[0053] (4) transfer the colostrum to a high-pressure homogenizer, and use 500bar pressure for high-pressure homogenization to obtain a submicron emulsion;
[0054] (5) Nitrogen-filled sub-package, after autoclaving...
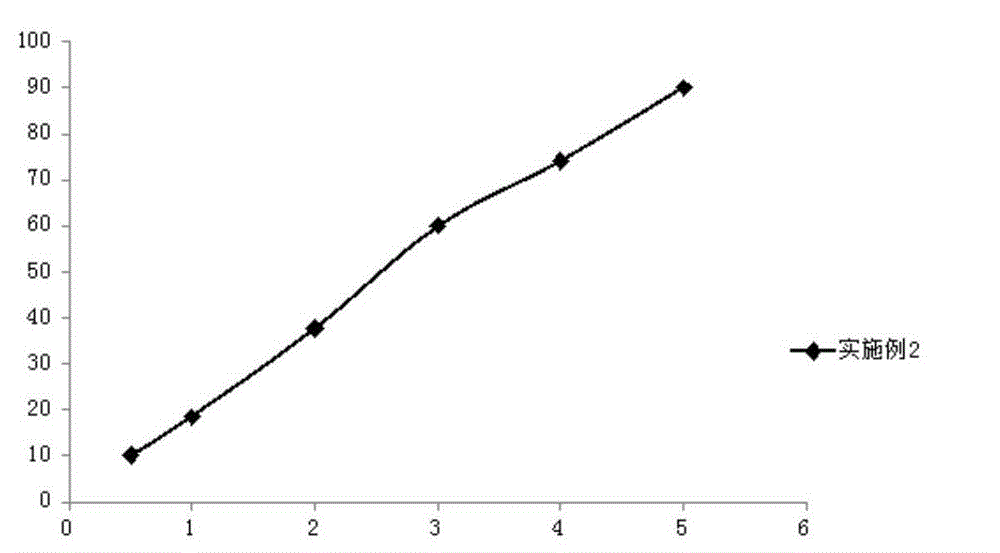
Embodiment 2
[0056] (1) Weigh 1.5g romidepsin, 6.5g soybean oil, 10.5g medium-chain fatty acid triglycerides, 2.0g egg yolk lecithin, 0.8g propylene glycol and 0.2g ethanol in a beaker, and use a high-speed shearing machine Shear at high speed for 5 minutes, heat and stir in a magnetic stirring water bath at 65°C at a stirring speed of 500rpm to completely dissolve it to obtain an oil phase;
[0057] (2) Weigh 0.9g of sodium chloride and put it in a beaker, add 77.4g of water for injection, heat and stir in a magnetic stirring water bath at 65°C, and stir at a speed of 400rpm, so that it is completely dissolved to obtain an aqueous phase;
[0058] (3) under high-speed stirring, the oil phase and the water phase are mixed, and the pH value is adjusted to 7.2 to obtain colostrum;
[0059] (4) transfer the colostrum to a high-pressure homogenizer, and use 350bar pressure for high-pressure homogenization to obtain a submicron emulsion;
Embodiment 3
[0062] (1) Weigh 2.5g romidepsin, 4.5g safflower oil, 15.5g medium-chain fatty acid triglycerides, 2.0g hydrogenated egg yolk lecithin, and 1.0g propylene glycol in a beaker, and use a high-speed shearing machine to perform high-speed shearing. Cut for 8 minutes, heat and stir at 45°C in a magnetic stirring water bath at a stirring speed of 700rpm to completely dissolve and obtain an oil phase;
[0063] (2) Weigh 4.5g of mannitol and place it in a beaker, add 70g of water for injection, heat and stir at 45°C in a magnetic stirring water bath at a stirring speed of 700rpm, and dissolve it completely to obtain an aqueous phase;
[0064] (3) under high-speed stirring, the oil phase and the water phase are mixed, and the pH value is adjusted to 6.7 to obtain colostrum;
[0065] (4) transfer the colostrum to a high-pressure homogenizer, and use 650bar pressure for high-pressure homogenization to obtain a submicron emulsion;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com