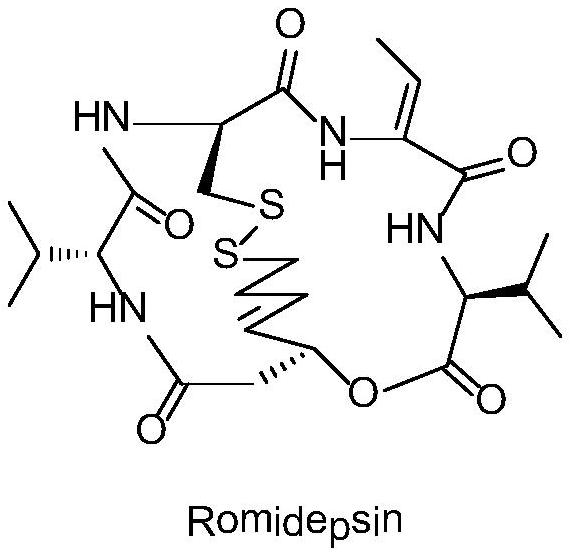
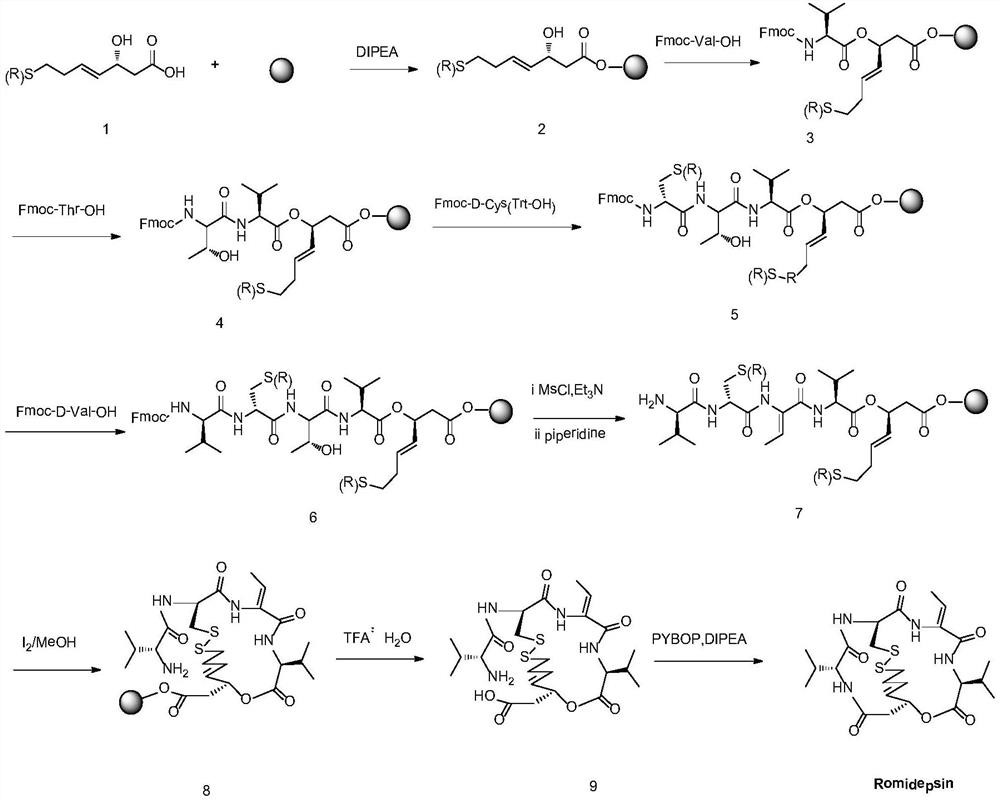
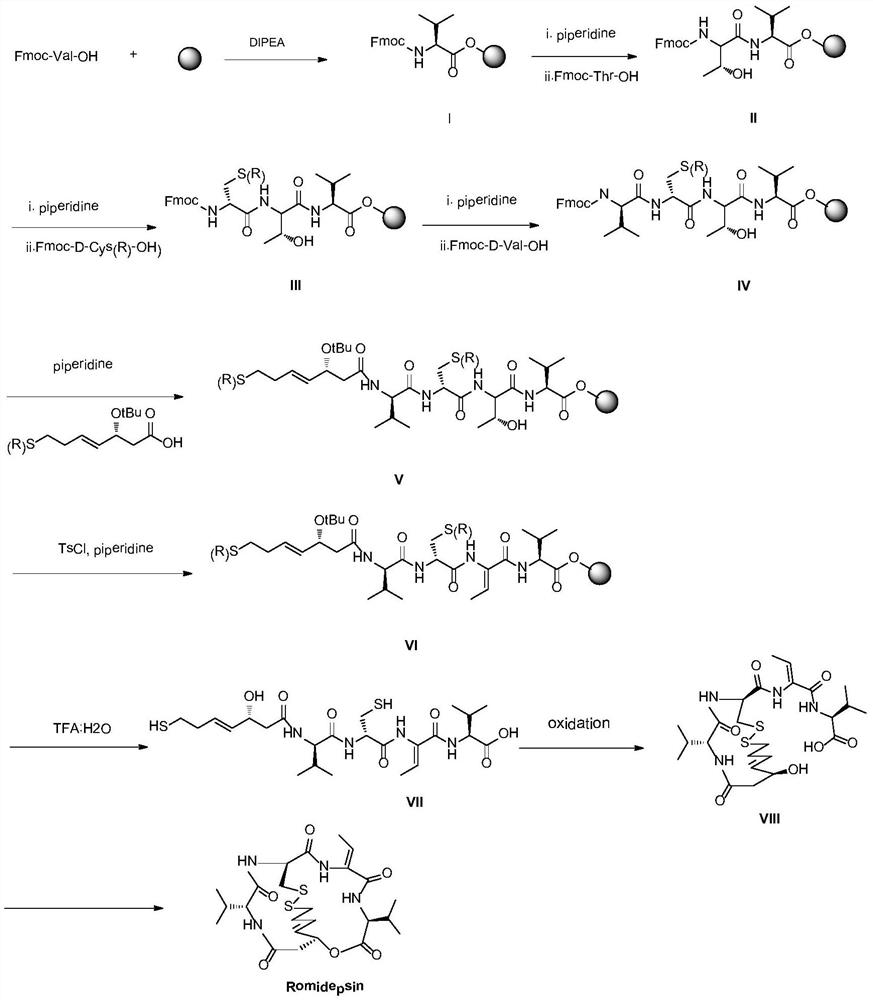
A kind of synthetic method of romidepsin
A synthesis method and technology for romidepsin, which are applied in the field of solid-phase synthesis of medicinal chemistry, can solve the problems of cumbersome steps, not simple enough, and affect the production efficiency of romidepsin, and achieve the effects of simple steps and high synthesis efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The preparation of embodiment 1 intermediate I
[0061] Weigh 2g of CTC Resin with a degree of substitution of 0.5mmol / g (synthesis scale 1mmol), add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, then weigh 1.01g of Fmoc-Val-OH , 0.38g HOBt and 0.03g DMAP were dissolved in DMF, activated by adding 0.6mL DIPEA in an ice-water bath, then added to the above-mentioned reaction column equipped with resin, reacted for 2 hours and the reaction was completed, washed 6 times with DMF to obtain intermediate I.
Embodiment 2
[0062] The preparation of embodiment 2 intermediate V
[0063] Use 20% DBLK to remove the Fmoc protecting group of intermediate I in the solid-phase reaction column, then wash it with DMF for 6 times, take a small amount of resin for ninhydrin test, and the resin will develop color. Weigh 1.02g Fmoc-Thr-OH, 0.38g HOBt, 0.03g DMAP and dissolve in a mixed solution of DMF and NMP with a volume ratio of 1:1, add 0.3mL DIC to activate it in an ice-water bath, add it to a solid-phase reaction column, and react at room temperature 2h (the end point of the reaction is determined by the ninhydrin method. If the resin is colorless and transparent, the reaction is complete, and the resin develops color, indicating that the reaction is incomplete, and another coupling reaction is required for 1h). Repeat the steps of removing Fmoc protection and adding the corresponding amino acid coupling steps to complete Fmoc-D-Cys(Trt)-OH, Fmoc-D-Val-OH and (R)-3-tert-butoxy-7-mercapto -4-Heptenoic a...
Embodiment 3
[0064] The preparation of embodiment 3 intermediate VI
[0065] Weigh 0.03g DMAP, 0.20g p-toluenesulfonyl chloride dissolved in 10mL dichloromethane, then add 0.60ml piperidine. Then the mixed solution was added to the solid-phase reaction column containing intermediate V, and reacted for 2 hours. After the reaction, it was washed with DMF for 6 times, then shrunk with methanol for 3 times, and vacuum-dried to obtain 3.0 g of intermediate VI.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com