Preparation method for synthesizing romidepsin dipolymer romipeptide A
A technique for synthesizing romidepsin and romidepsin, which is applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., and can solve the problem of low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Compound Synthesis
[0018] 1.0 g of romidepsin raw material was dissolved in 50 mL of acetone, then 1.0 L of NaOH (0.01 M, added dropwise in 20 minutes) was added and the reaction was stirred until the romidepsin reaction was completed and the reaction was terminated.
[0019] The reaction solution was extracted twice with 2L of ethyl acetate to obtain an ethyl acetate phase, which was washed with water until neutral, dried with anhydrous sodium sulfate and concentrated to dryness, and then separated by semi-preparative column chromatography to obtain romidepsin dimerization. compound (compound 1). The semi-preparative column chromatography conditions are as follows:
[0020] The chromatographic column is: Eclipse XDB-C18, 9.4mm×250mm;
[0021] The mobile phase is: methanol: water = 75:25;
[0022] Detection wavelength: 210nm;
[0023] Flow rate: 1.5ml / min;
[0024] Column temperature: room temperature
[0025] Retention time (weight): 13.4 mins (216 mg...
Embodiment 2
[0026] Example 2 Structure identification
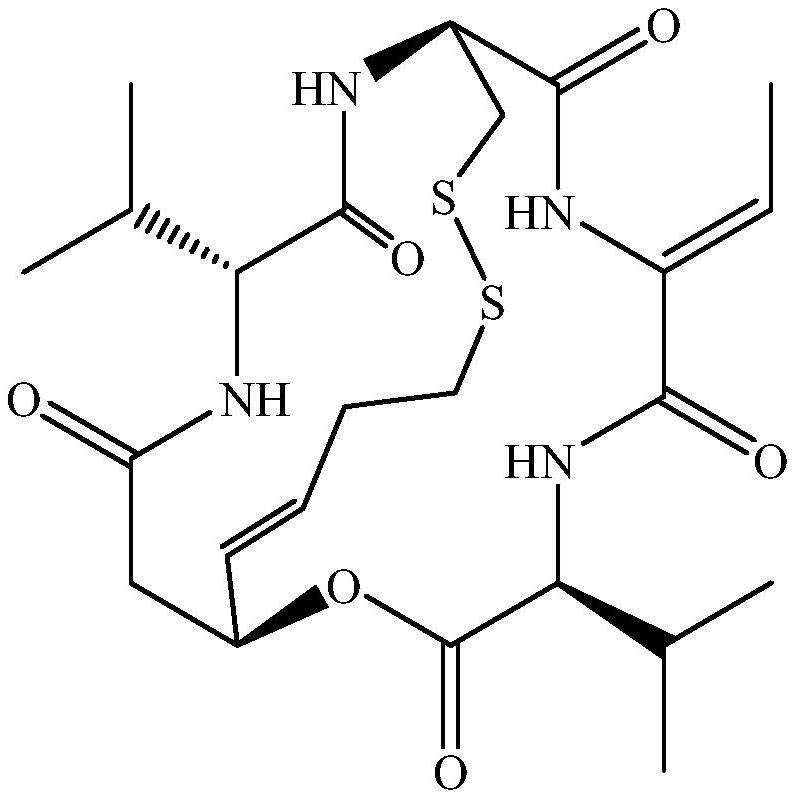
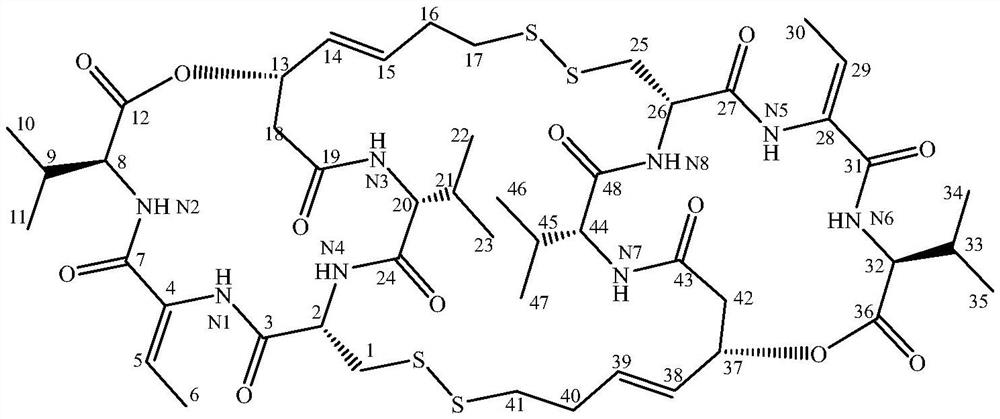
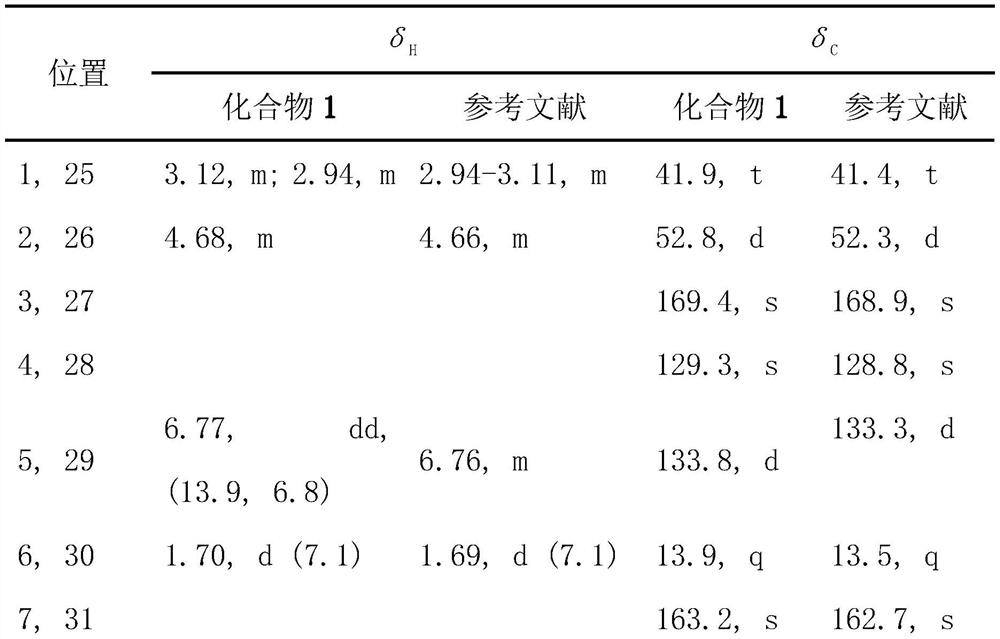
[0027] Compound 1 obtained in Example 1, (c 0.07, EtOH), UV(EtOH) λmax nm(logε): 202(3.45).HRESIMS m / z 1081.4221[M+H] + (C 48 H 73 N 8 O 12 S 4 The calculated value is 1081.4231). 1 H and 13 CNMR data and references (Xiong L, Chen Ca, Min TL, Hu HF. Romipeptides A and B, two newromidepsin derivatives isolated from Chromobacterium violaceum No.968 and their antitumor activities in vitro. Chin J Nat Med, 2019, 17(2): 155-160.) The comparison is shown in Table 1. In summary, compound 1 is romipeptide A, and the structure is shown in formula 2.
[0028] Table 1 Nuclear magnetic data (DMSO-d6) of the dimer obtained in Example 1
[0029]
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com