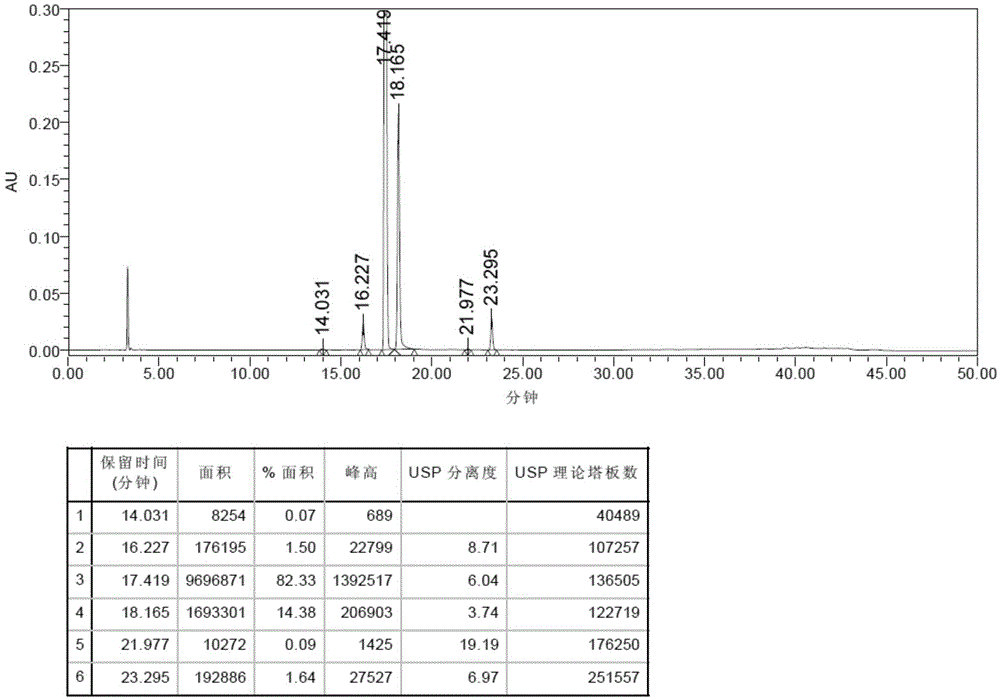
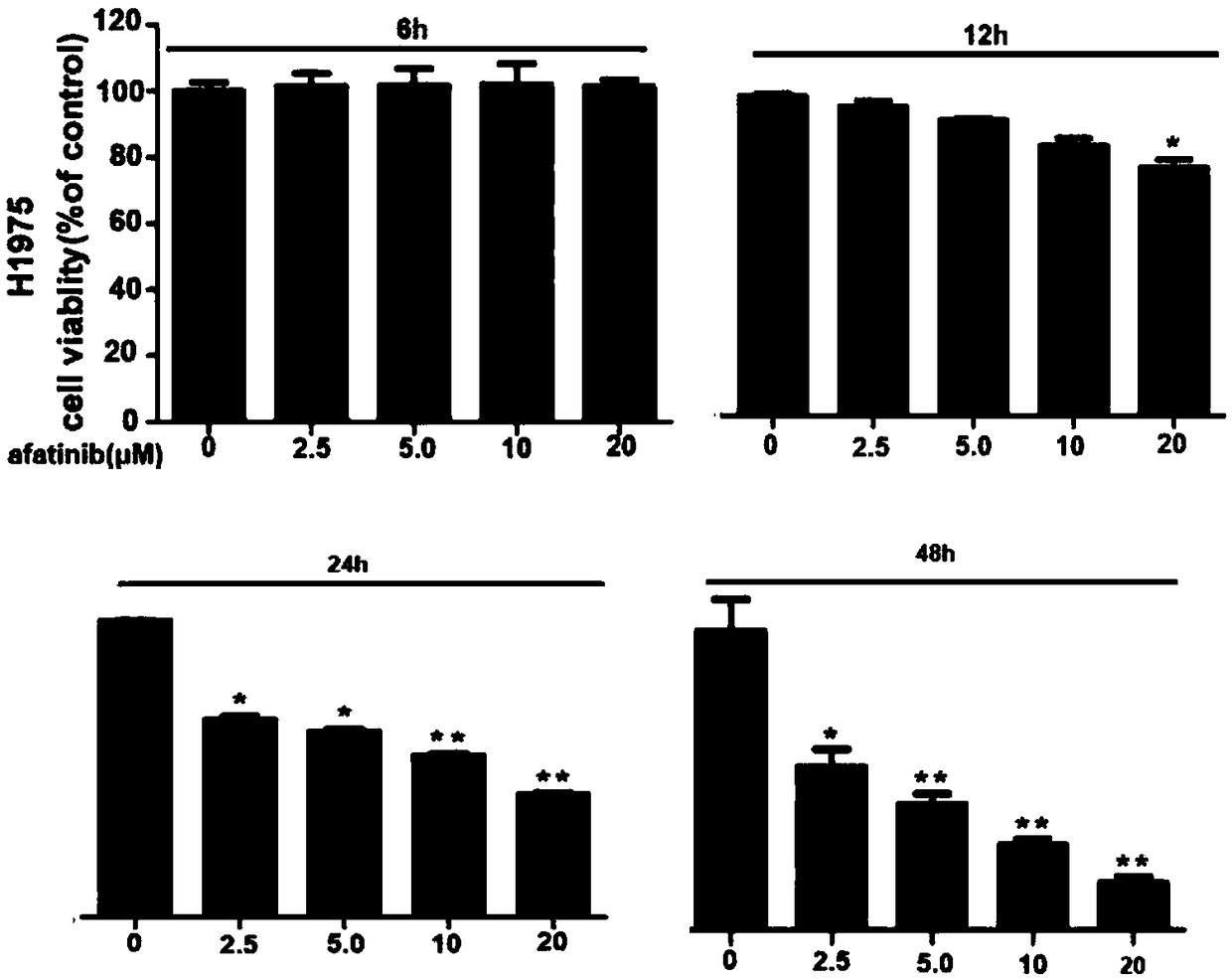
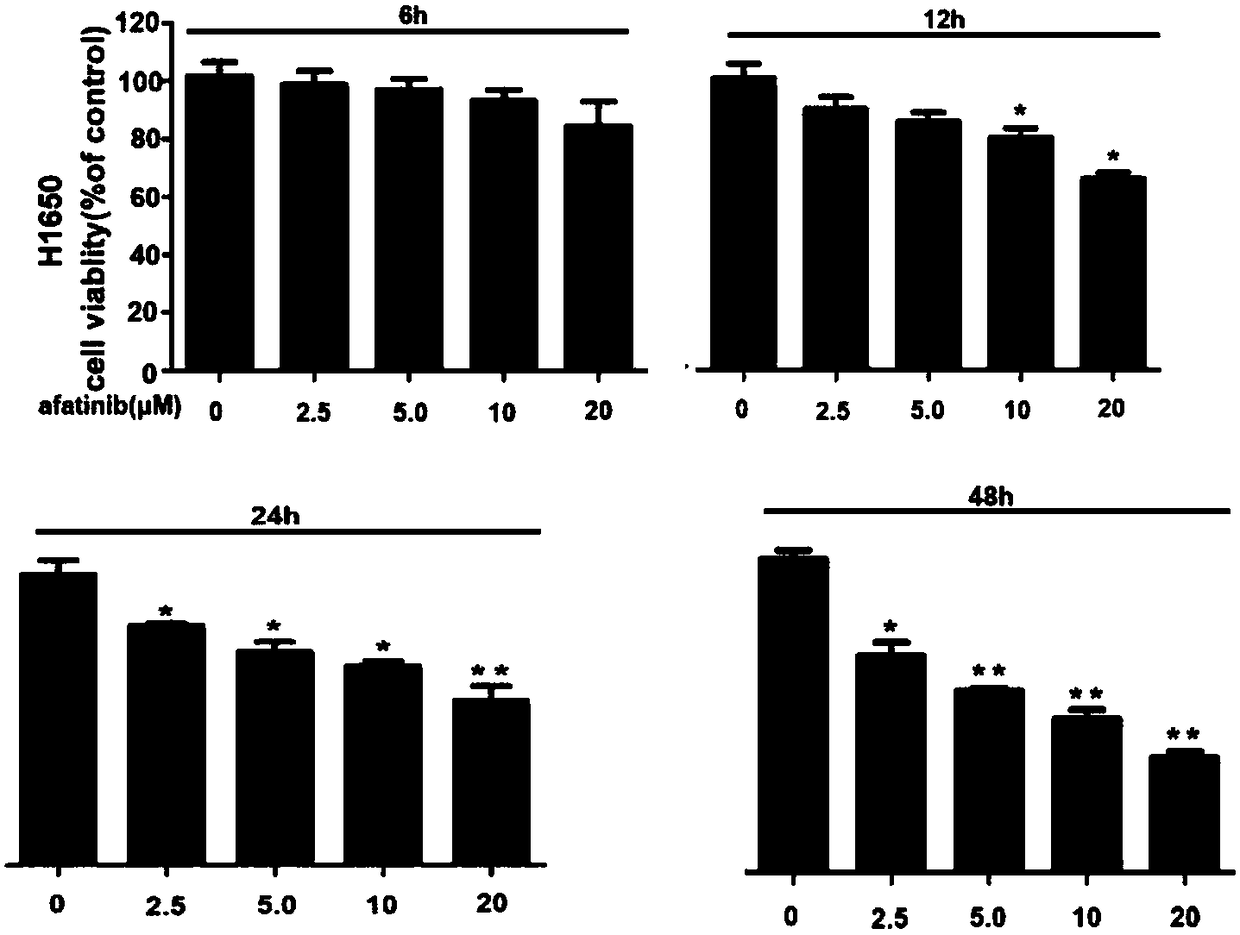
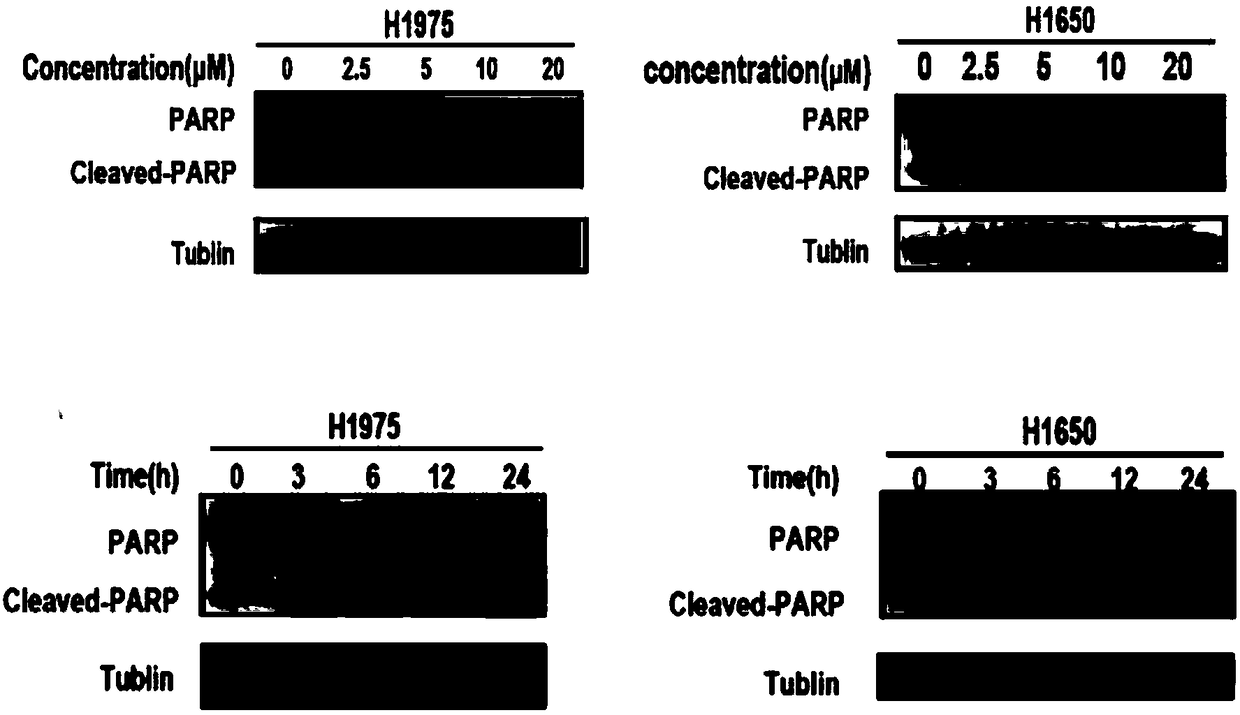
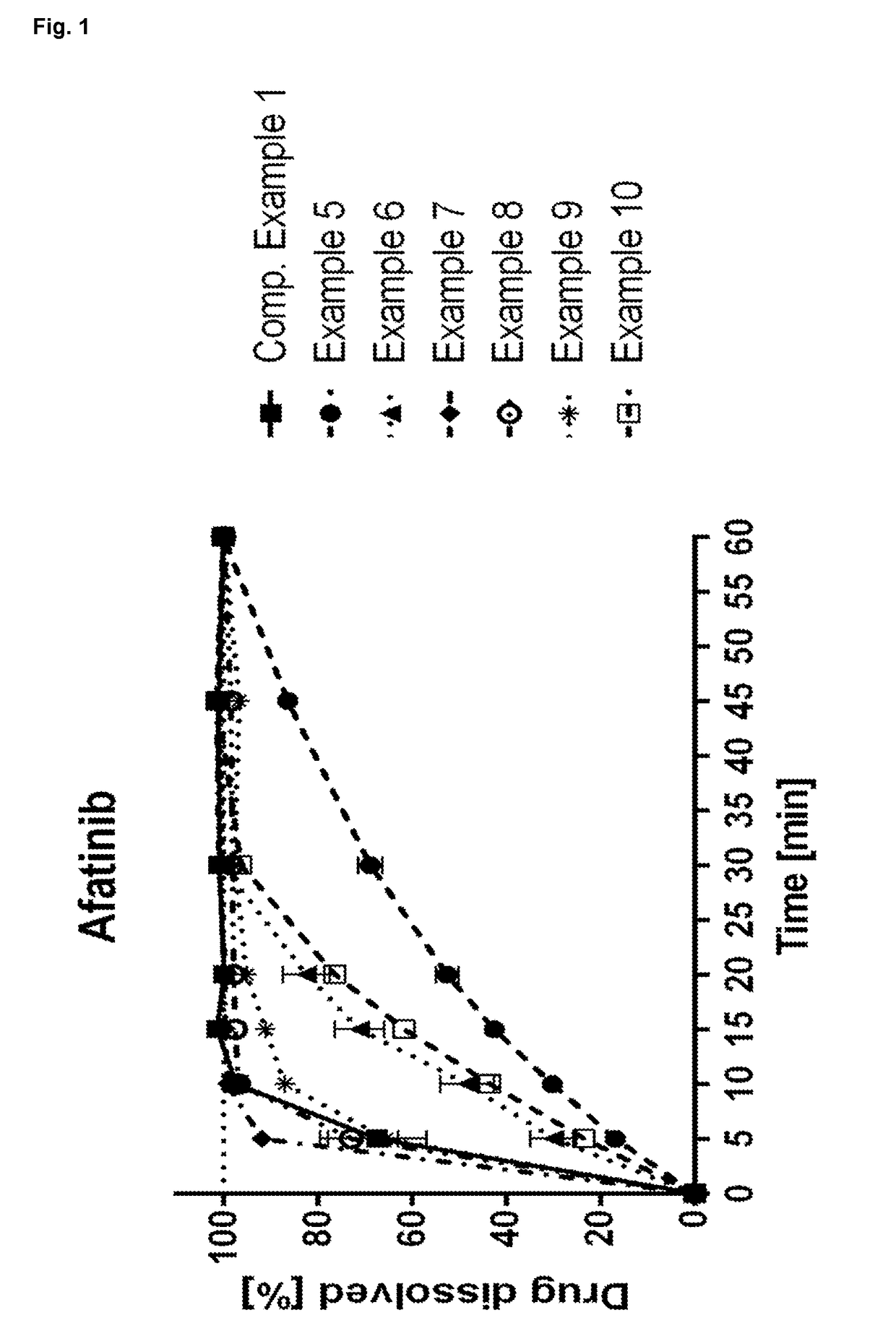
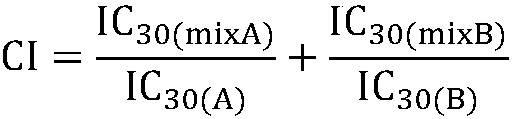Patents
Literature
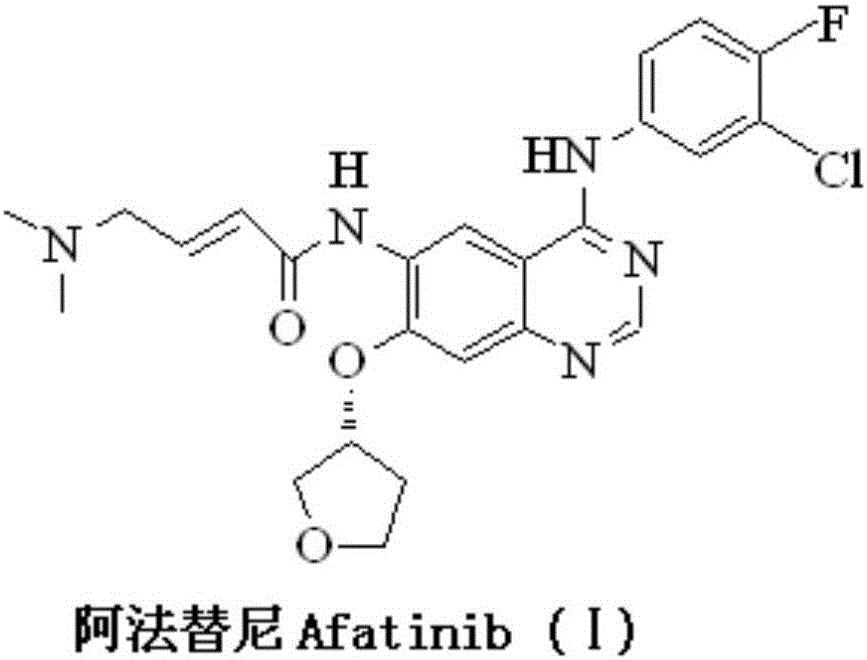
120 results about "Afatinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
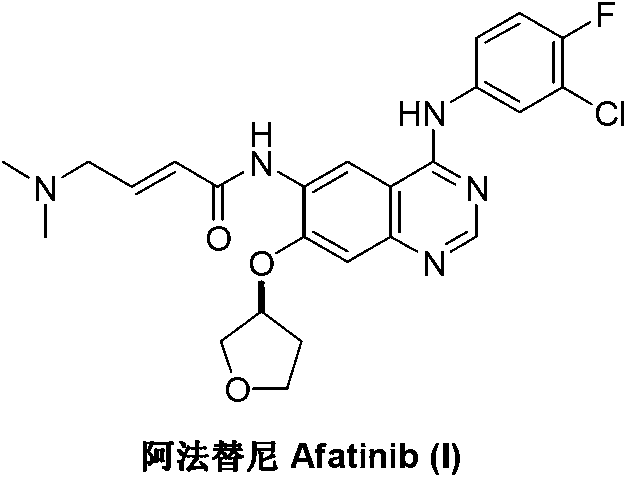
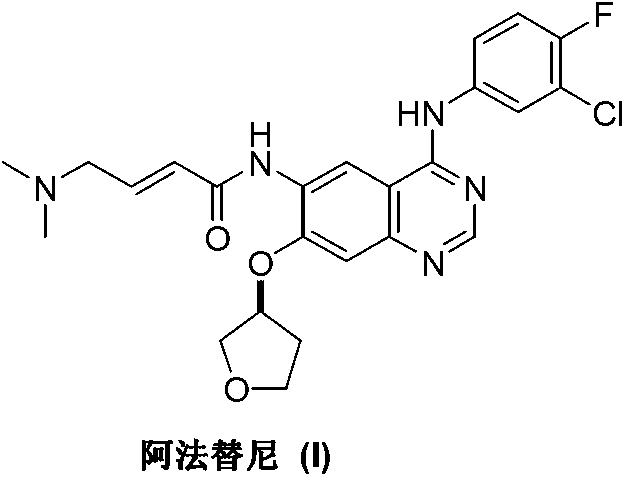
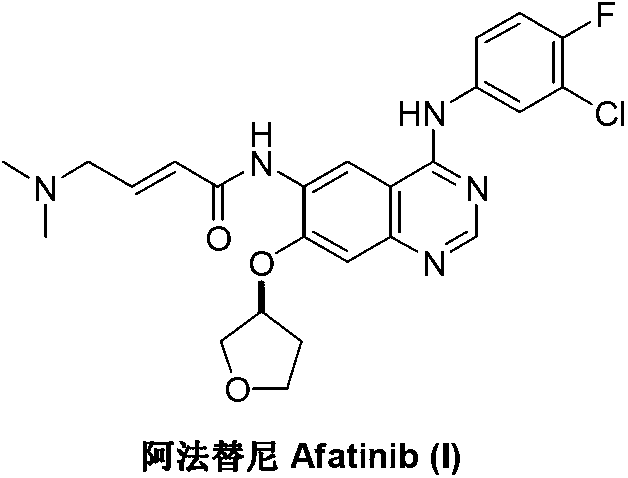
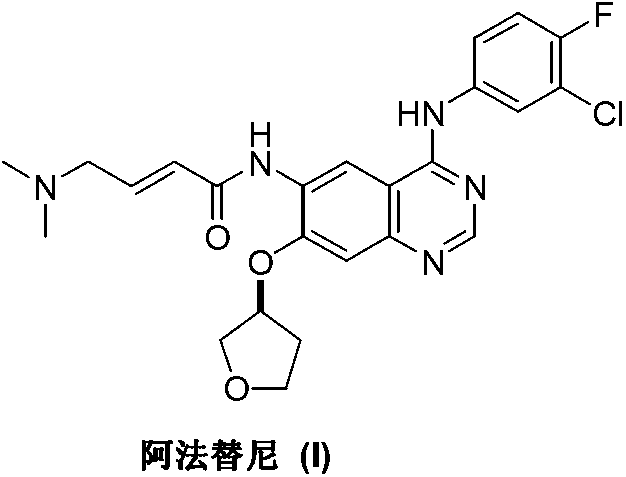
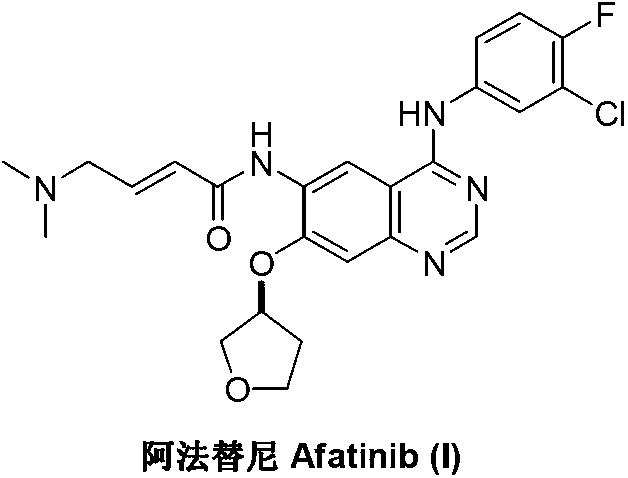
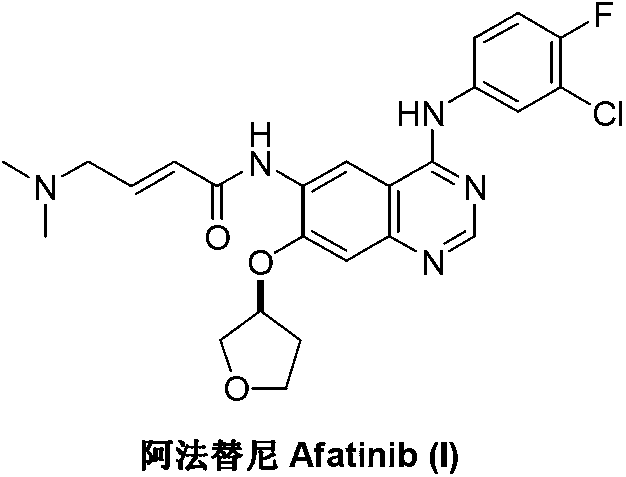
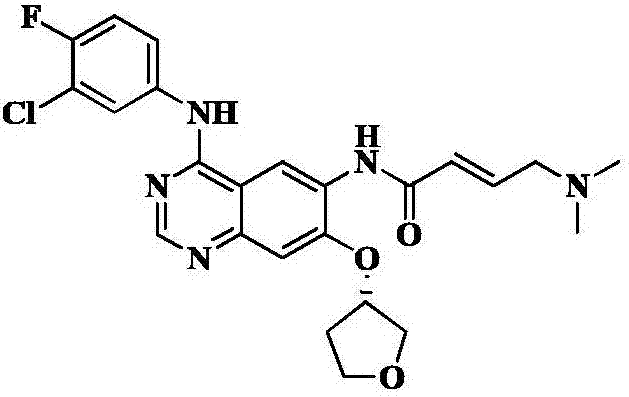
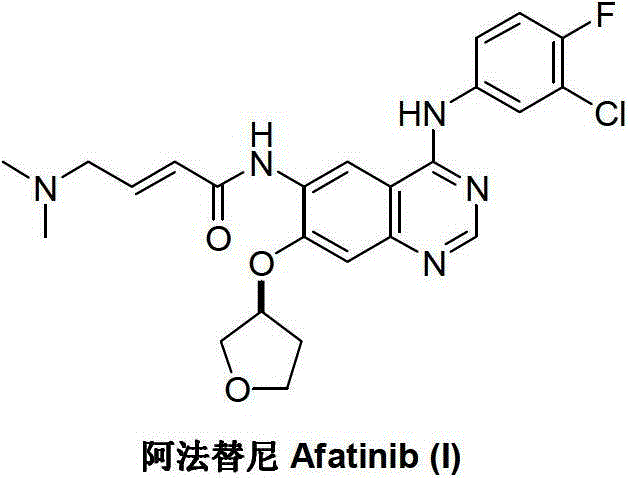
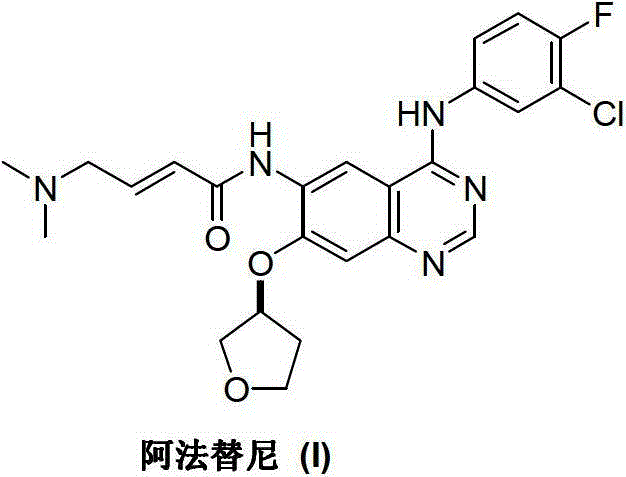
Afatinib is used to treat a certain type of lung cancer (non-small cell lung cancer) that has spread to other parts of the body.
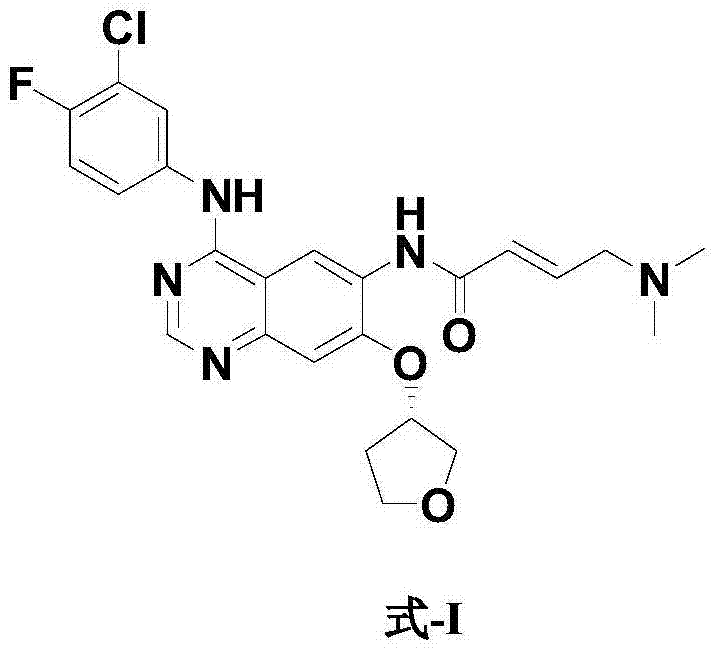
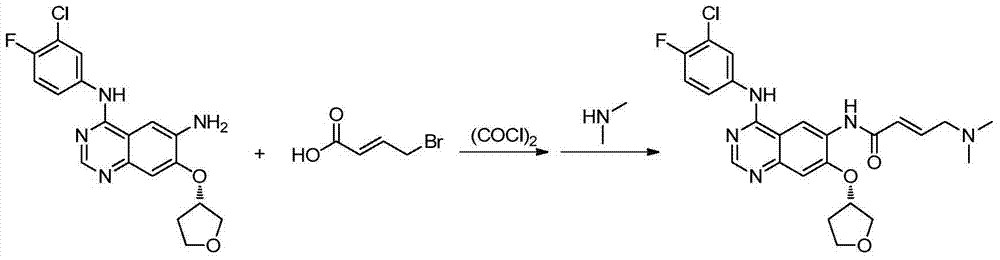
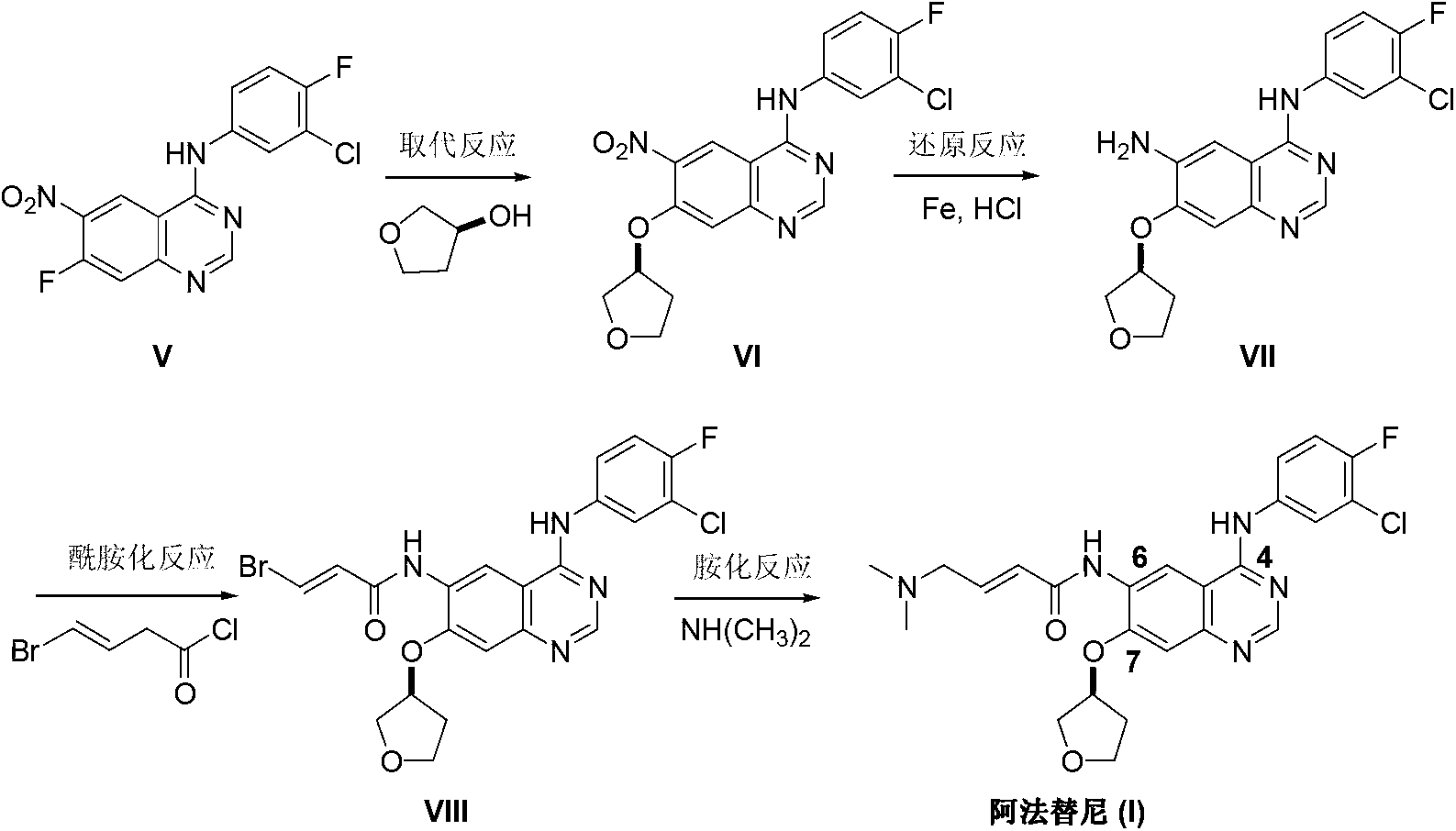
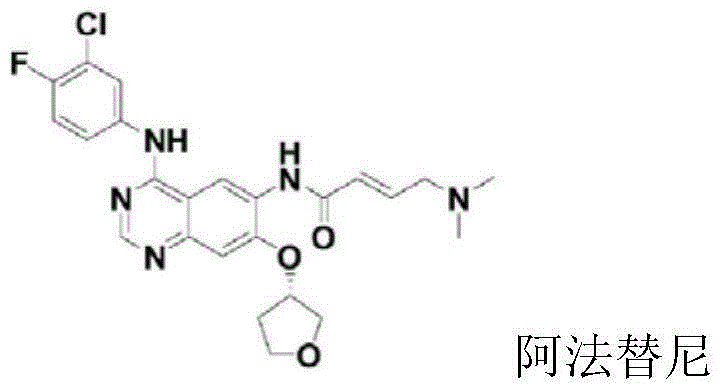
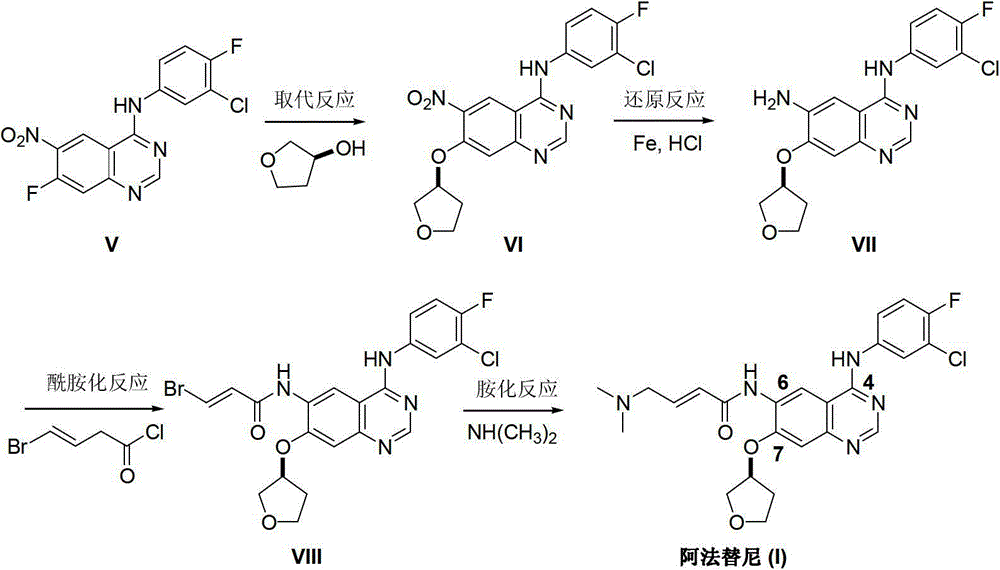
Preparation method for afatinib compound
InactiveCN103755688AReduce pollutionRaw materials are easy to getOrganic chemistrySynthesis methodsQuinazoline
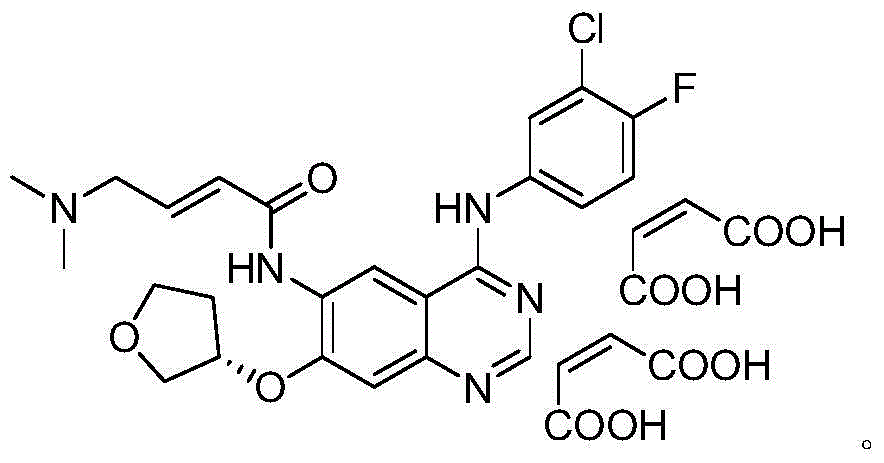
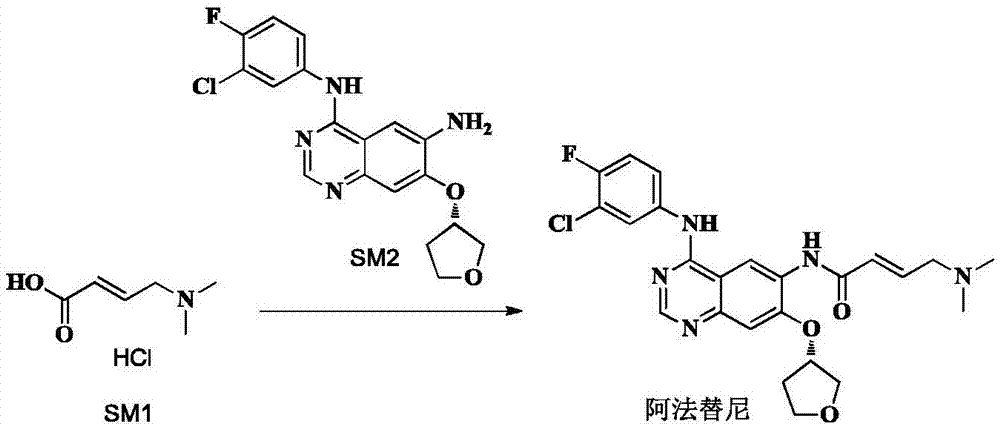
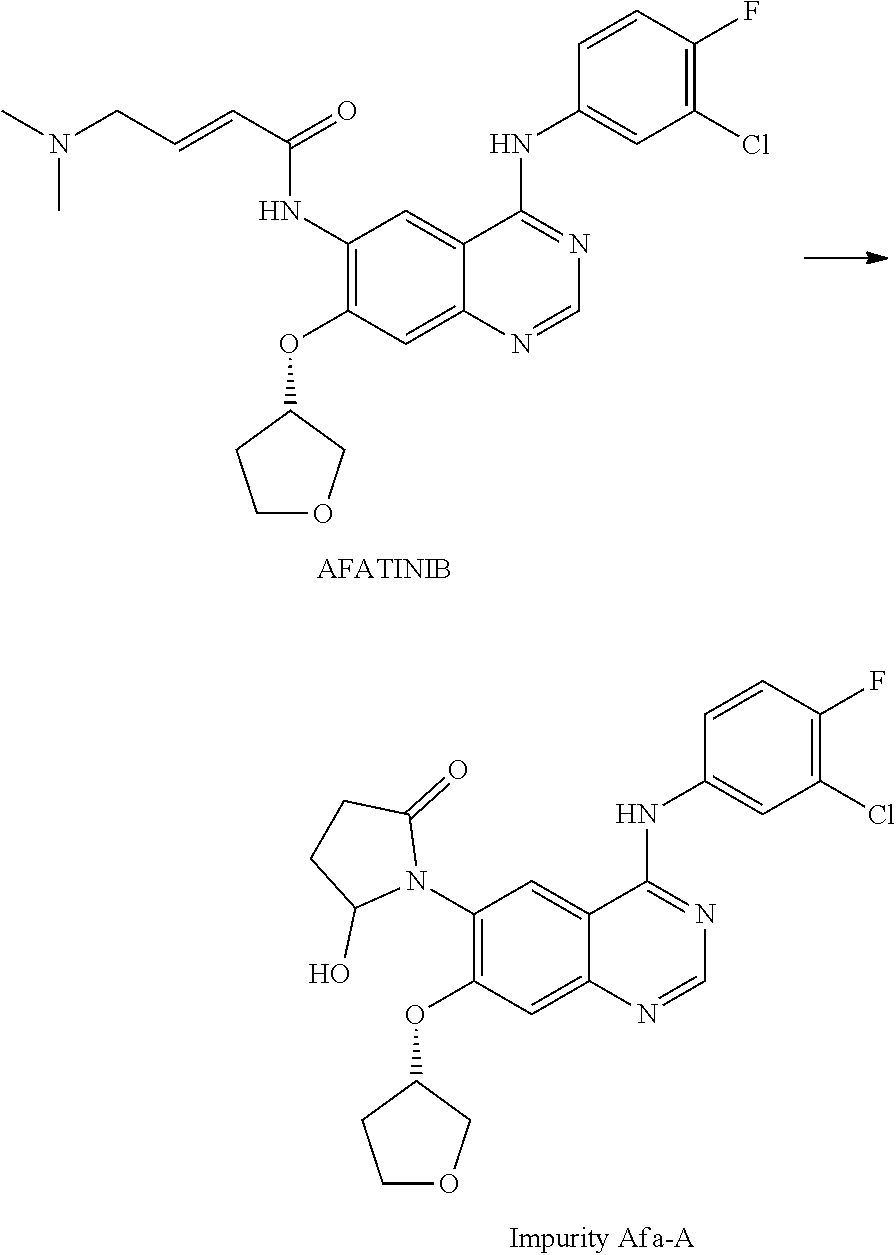
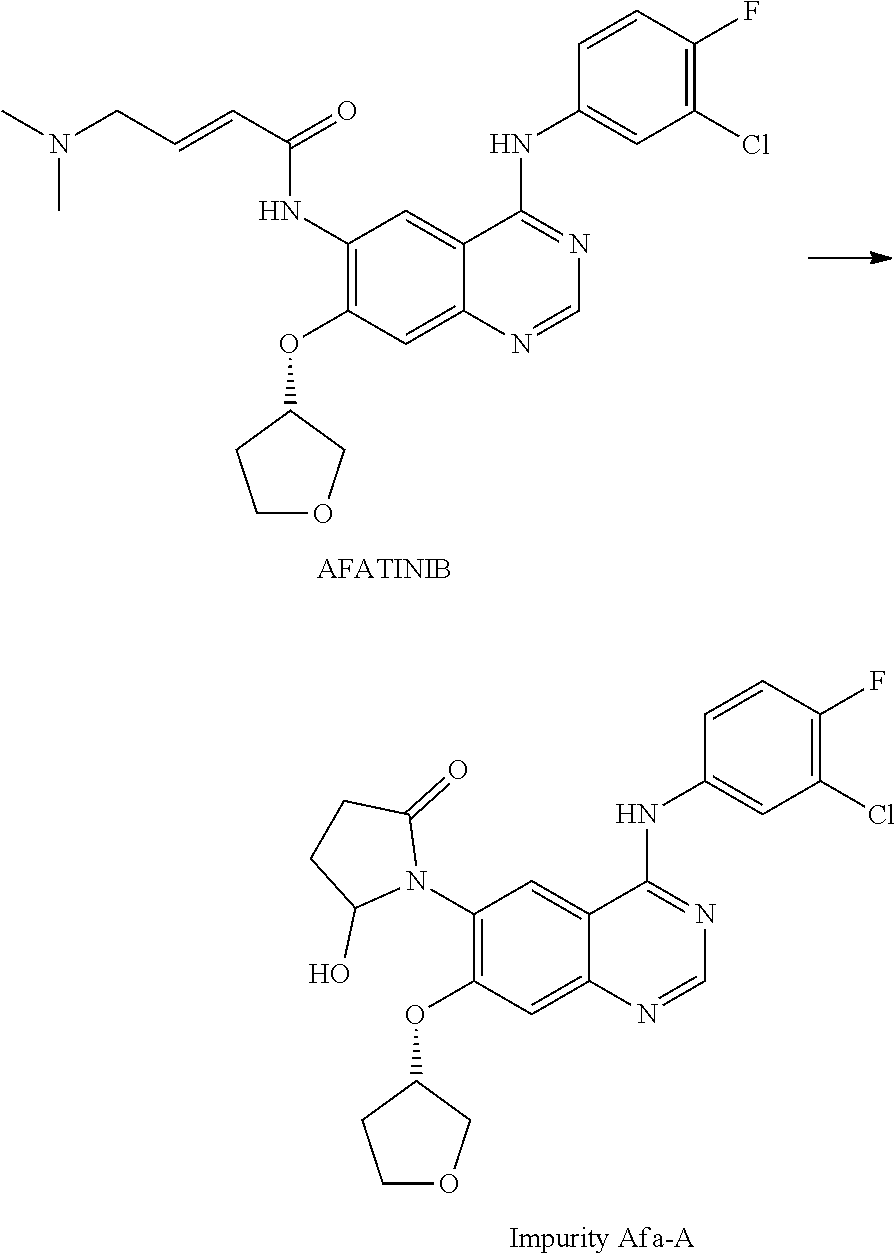
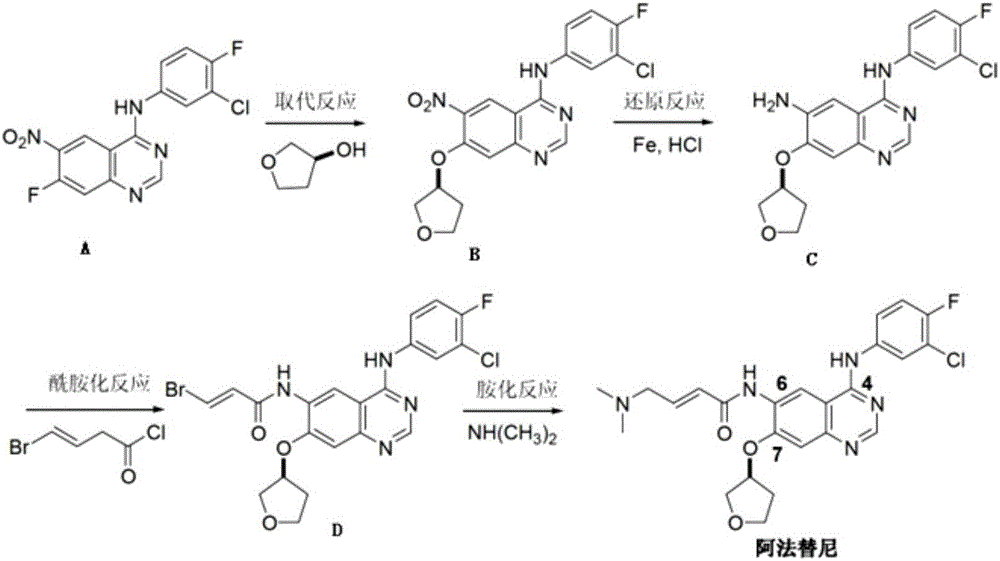
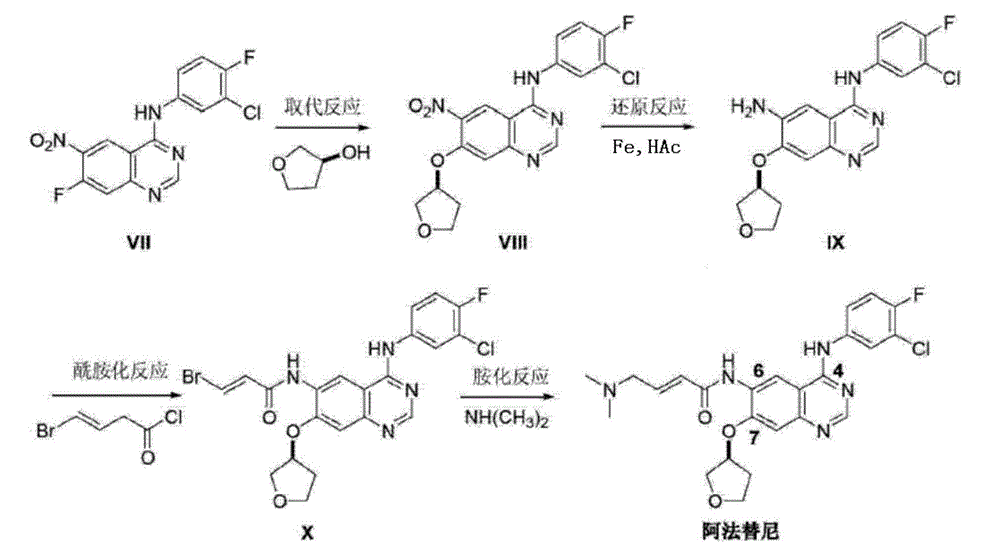
The invention provides a novel synthesis method for afatinib. The afatinib is prepared by activating or chlorinating dimethylamino crotonic acid hydrochloride by using an amide connection reagent and then reacting with N<4>-(3-chloro-4-fluoro-phenyl)-7-((S)-tetrahydrofuran-3-oxy)quinazoline-4,6-diamine. The invention further provides a process of preparing dimethylamino crotonic acid hydrochloride and an intermediate thereof. The method for preparing the afatinib provided by the invention has the advantages of low production cost, small environmental pollution, and simplicity and convenience in operation and is suitable for industrial production.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Eutectic complex composed of resveratrol and protein kinase inhibitor, and composition comprising eutectic complex
InactiveCN110283052AHydroxy compound active ingredientsOrganic chemistry methodsLenvatinibPTK Inhibitors
The invention provides a eutectic complex composed of resveratrol and a protein kinase inhibitor, and a composition comprising the eutectic complex. The eutectic complex is characterized in that the protein kinase inhibitor is selected from one of imatinib, gefitinib, erlotinib, sunitinib, sorafenib, dasatinib, lapatinib, nilotinib, pazopanib, afatinib, crizotinib, axitinib, regorafenib, ibrutinib, lenvatinib, palbociclib, osimertinib and alectinib or pharmaceutically acceptable salt thereof. The eutectic complex provided by the invention can produce synergistic effects of inhibiting histamine release.
Owner:黄泳华
Detection method of afatinib and isomer of afatinib
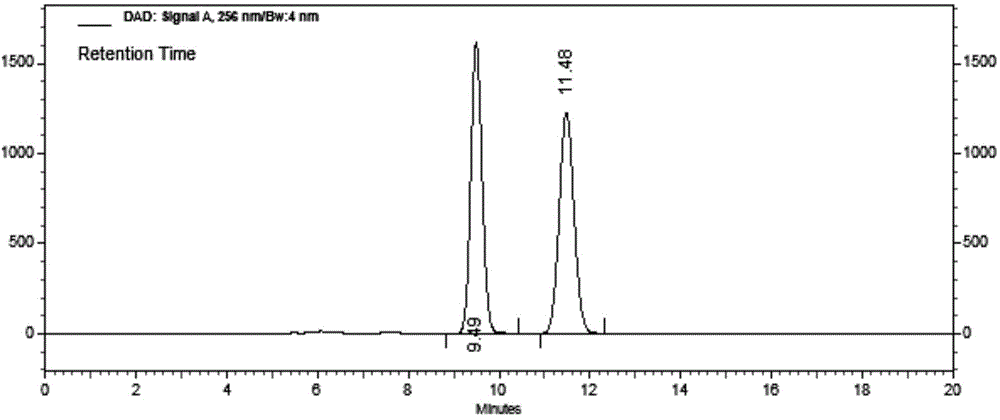
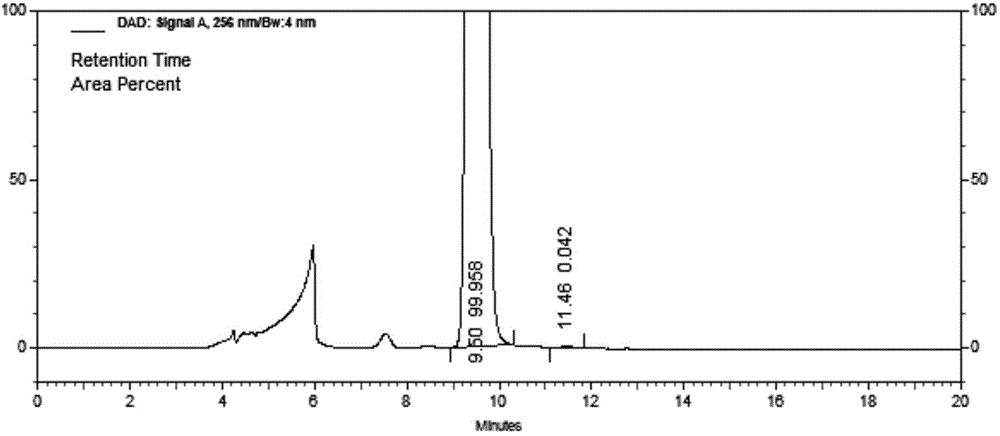
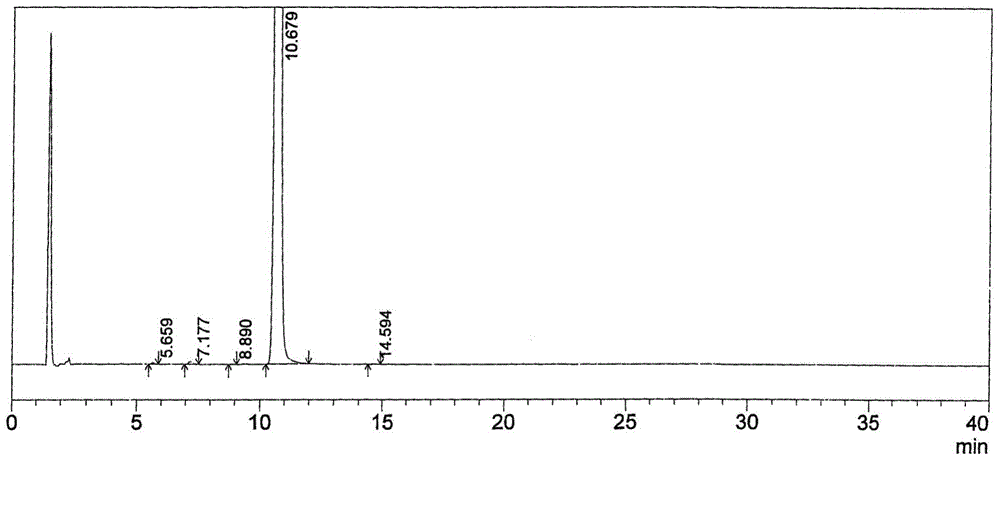
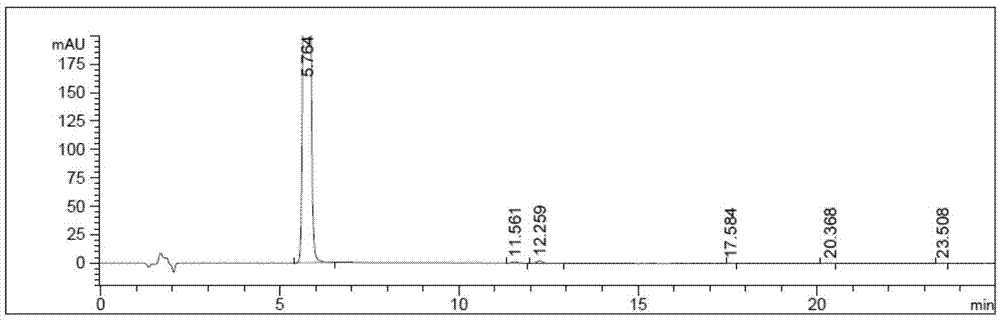
The invention relates to a liquid phase separation detection method of afatinib and enantiomer of the afatinib, and belongs to the technical field of separation detection. The method comprises the step of carrying out isocratic elution by adopting 250mm*4.6mm and 5 micrometers CHIRALPAK OZ-H chiral chromatographic column as a separation chromatographic column and adopting a mixed solution of n-hexane, ethanol, methanol and diethylamine as a flow phase. By adopting the method, the afatinib and an enantiomer impurity of the afatinib can be effectively separated and detected, and the peak pattern of a chromatographic peak is good.
Owner:SUNSHINE LAKE PHARM CO LTD
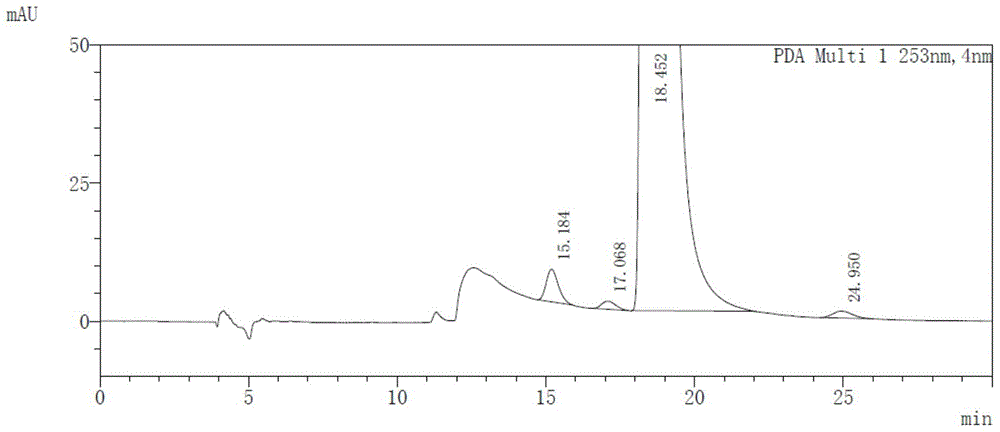
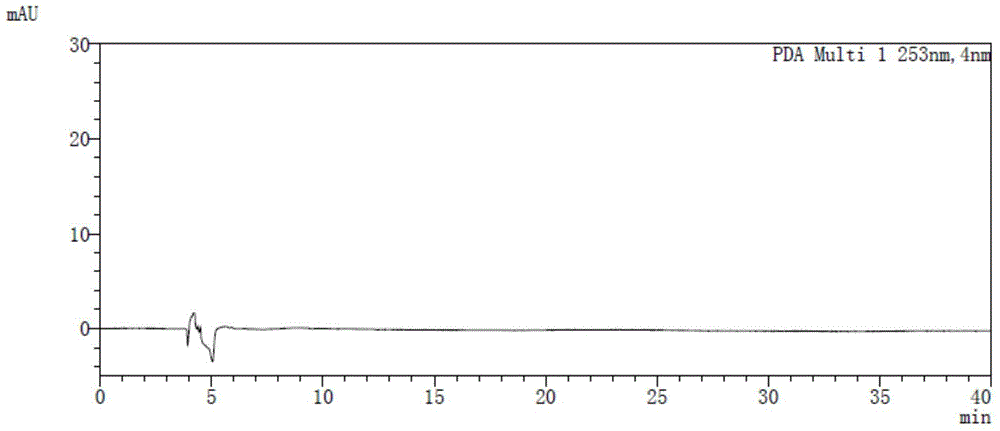
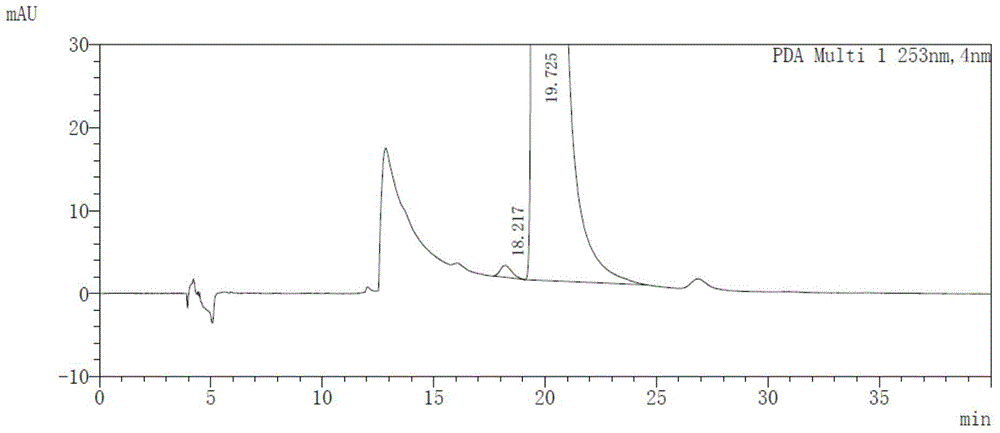
Method for detecting afatinib dimaleate isomers and main degradation impurities through high performance liquid chromatography
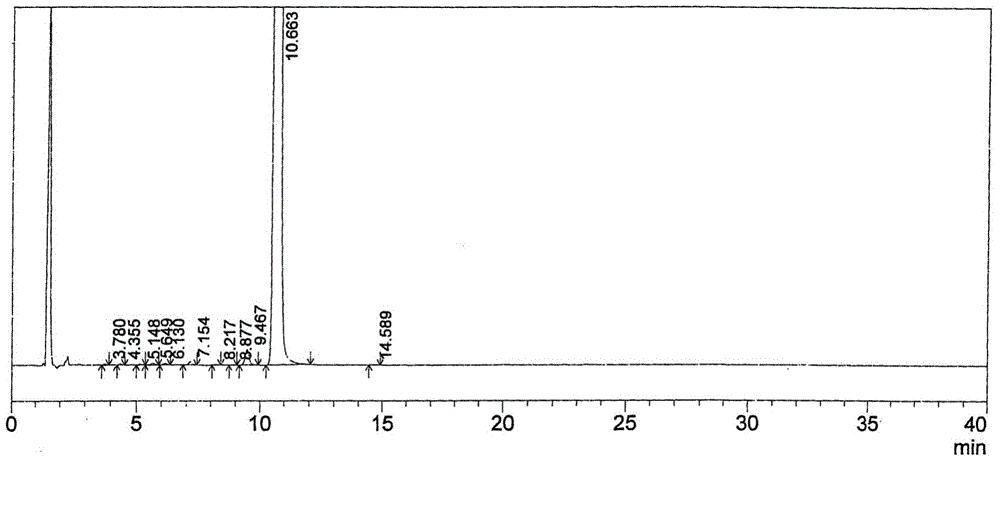
The invention relates to the technical field of analytical chemistry and discloses a method for detecting afatinib dimaleate isomer and main degradation impurities through a high performance liquid chromatography.According to the method, afatinib dimaleate raw materials or preparations are compounded into a detection solution with diluent, bonding amylose-trichlorobenzene carbamic acid ester serves as a stationary phase, a normal hexane-ethanol-organic base solution serves as a mobile phase for isocratic elution, and an HPLC spectrogram is recorded under afatinib absorption wavelength.According to the method, a chromatographic column with trichlorobenzene carbamic acid ester serving as a filling material, the normal hexane-ethanol-organic base solution serves as the mobile phase, the content of isomers existing in afatinib dimaleate and preparations thereof can be measured quantitatively, afatinib, the isomers and the main impurities can be separated completely, the peak pattern is good, the method meets the specifications in Chinese Pharmacopoeia, quality of afatinib dimaleate and afatinib dimaleate preparation products is effectively controlled, and the method is great in specificity, high in sensitivity and good in accuracy.
Owner:BEIJING COLLAB PHARMA
Medicinal composition for resisting non-small cell lung cancer, and application thereof
InactiveCN106668866ASolve the problem of partial drug resistanceInhibition of phosphorylation levelsOrganic active ingredientsAntineoplastic agentsActive componentDihydroartemisinin
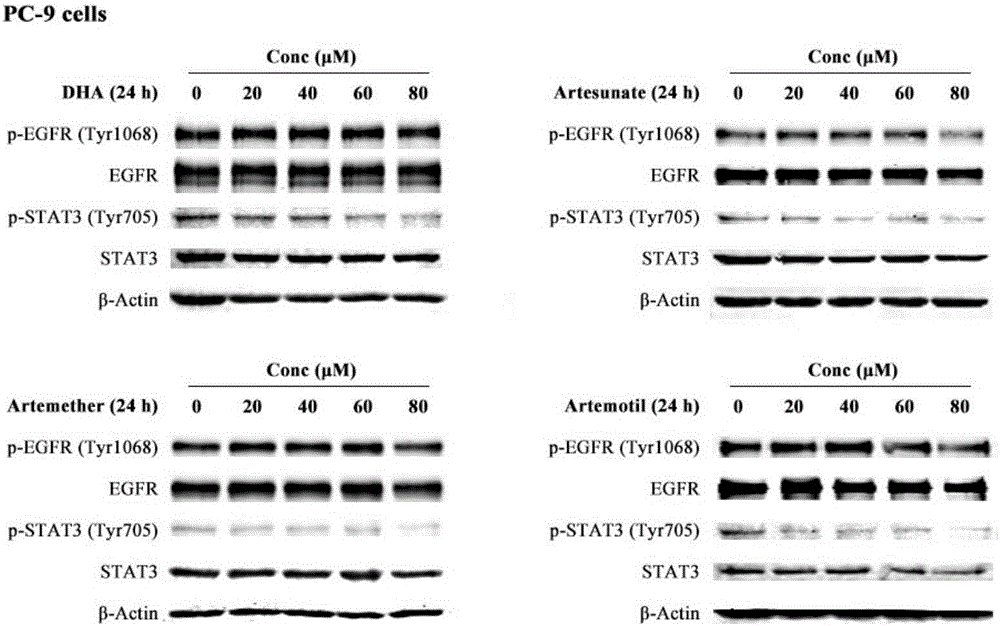
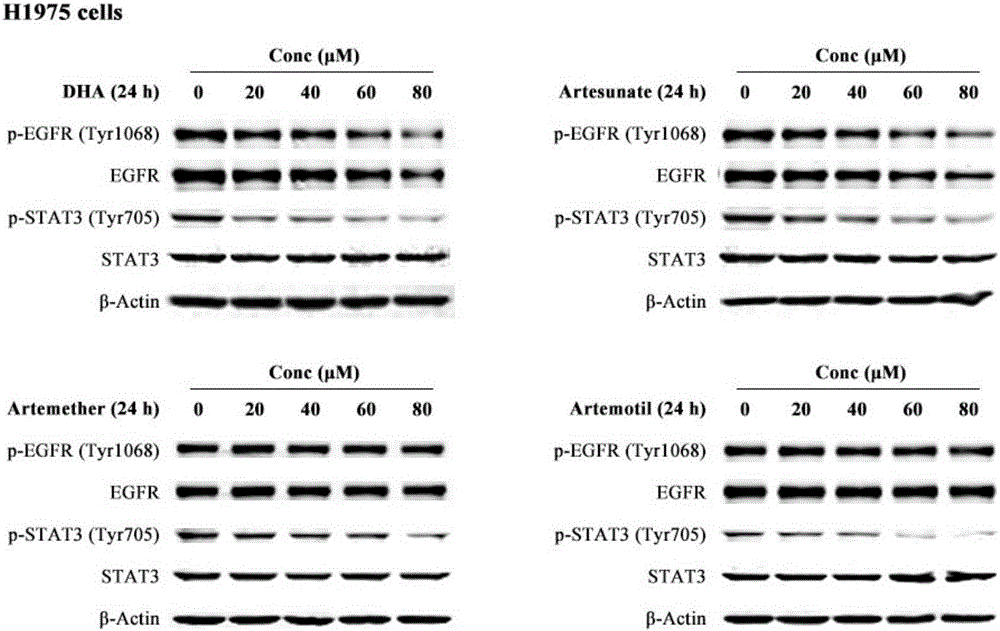
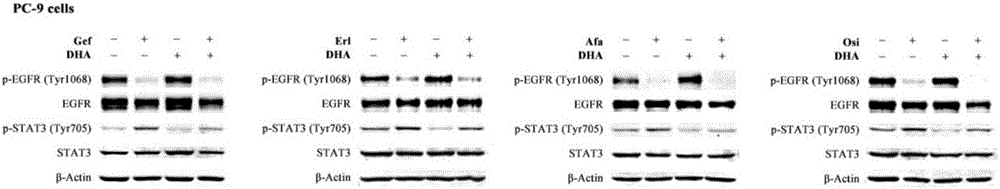
The invention discloses a medicinal composition for resisting non-small cell lung cancer. The active components of the medicinal composition comprise an artemisinin derivative and EGFR-TKI (epidermal growth factor receptor-tyrosine kinase inhibitor), wherein the artemisinin derivative is selected from one of dihydroartemisinin, artesunate, artemether and arteether; and the EGFR-TKI is selected from one of gefitinib, erlotinib, afatinib and osimertinib. The invention also discloses application of the medicinal composition to preparation of medicines for treating and resisting the non-small cell lung cancer. When the medicinal composition provided by the invention is used for treating the non-small cell lung cancer, the medicinal effect which is more excellent than that of the singly used EGFR-TKI can be achieved, the sensitizing effect is achieved, the problem of partial medicine resistance of the non-small cell lung cancer EGFR-TKI is solved, and a scientific basis is provided for the development of new medicines.
Owner:JIANGSU PROVINCE INST OF TRADITIONAL CHINESE MEDICINE
Preparation method of afatinib compound
The invention provides a novel preparation method of an afatinib compound. Raw materials and reagents used in the preparation method have the advantages of low cost, stable chemical property and convenience in long-term storage and the content of an impurity cis-isomer in the prepared afatinib compound is very low.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD +1
Afatinib preparation method
ActiveCN103242303AEase of industrial productionPromote the development of economy and technologyOrganic chemistry3-Hydroxytetrahydrofuran2-Butene
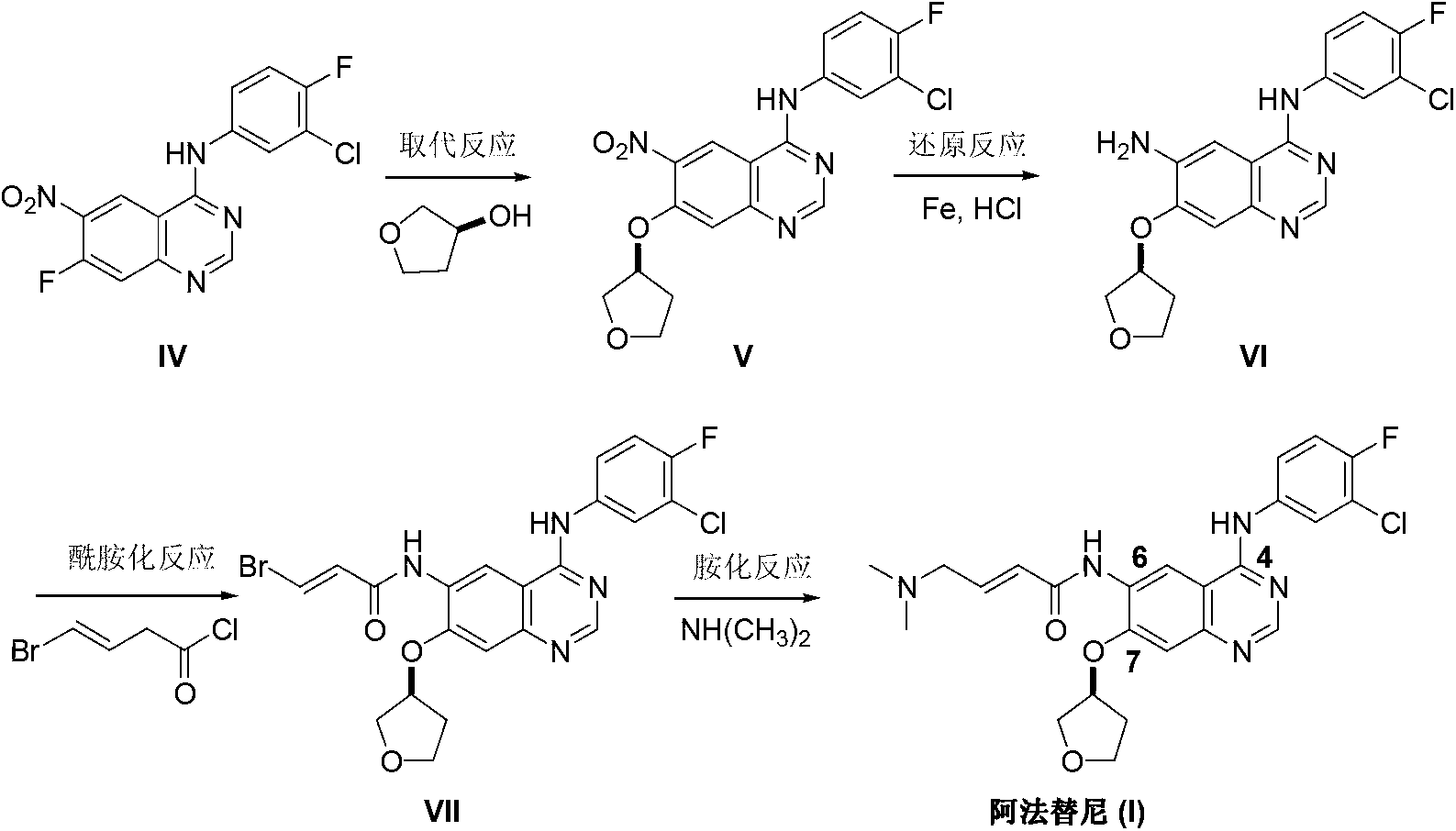
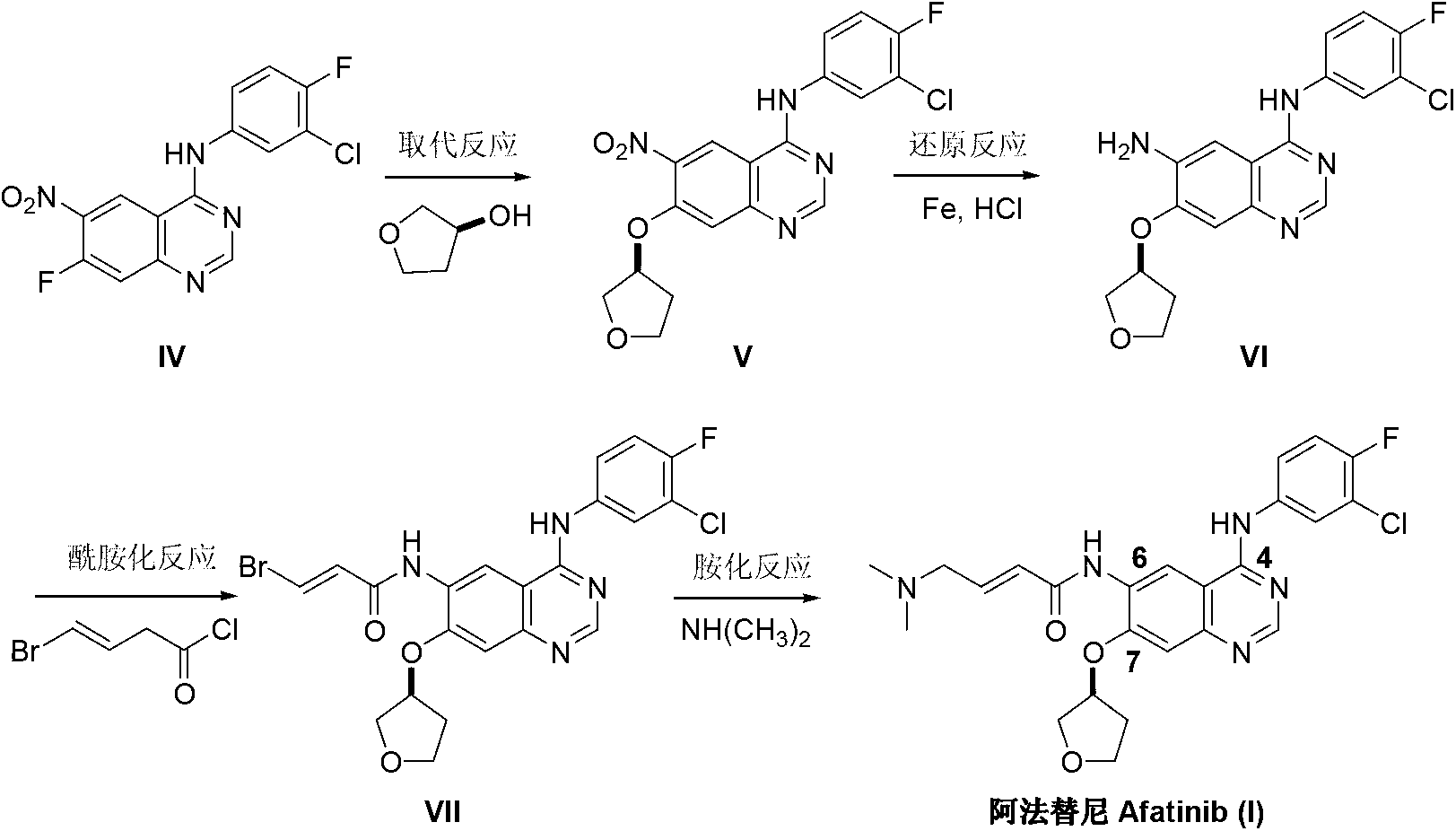
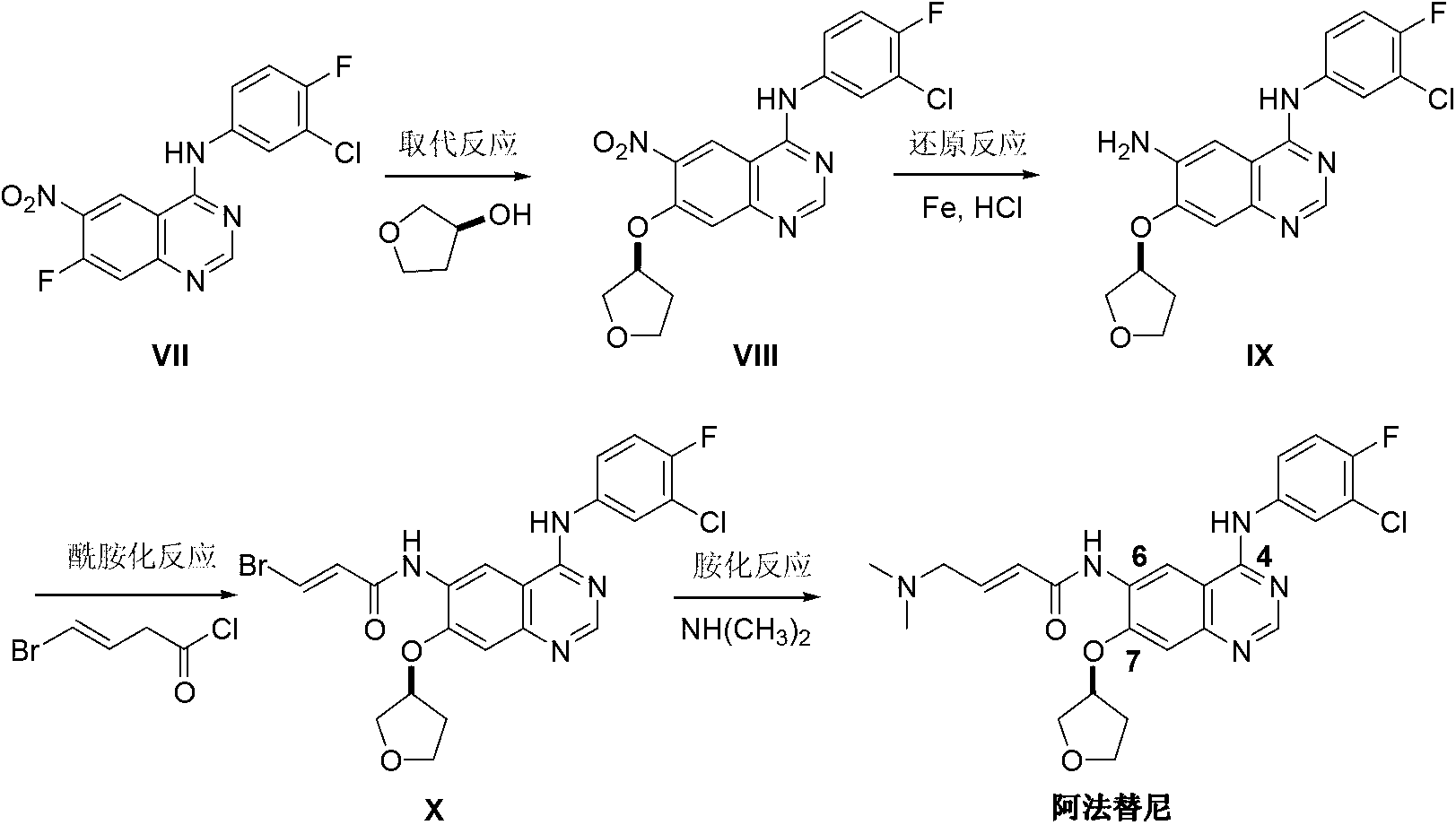
The invention discloses an Afatinib (I) preparation method which comprises the following steps: performing etherification reaction on 4-chloro-6-amino-7-hydroxyquinazoline (II) and (S)-3-hydroxytetrahydrofuran to generate 4-chloro-6-amino-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline (III); performing acylation reaction on the compound (III) and 4-(N,N-dimethylamino)-2-ene-butyryl chloride to generate 4-chloro-6-{[4-(N,N-dimethylamino)-1-oxo-2-butene-1-yl]amino}-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline (IV); and performing condensation reaction on the compound (IV) and 4-fluoro-3-chloroaniline to obtain Afatinib (I). The preparation method is simple, economic and environment-friendly in process, and meets the requirements for large-scale industrialization.
Owner:铜陵尚东高新科创有限公司
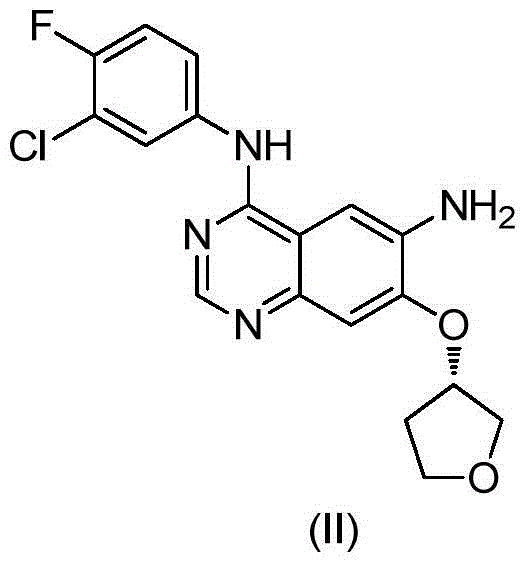
High purity preparation method of Afatinib intermediate
The present invention relates to a high purity preparation method of Afatinib intermediate, and particularly relates to a high purity preparation method of an antineoplastic treatment medicine maleate Afatinib intermediate (II) compound, the method comprises the following steps: an objective product is obtained sequentially through substitution in two steps, reduction reaction and the like of 6-amino-7-fluoro-3,4-dihydro-quinazolin-4-one. The method is simple in process, economic and environmental-friendly, and suitable for industrial amplification requirements, and the manufactured finished product is high in purity.
Owner:JIANGSU HANSOH PHARMA CO LTD
Method for detecting afatinib-dimaleate-related substances through high performance liquid chromatography
The invention relates to the technical field of analytical chemistry and discloses a method for detecting afatinib-dimaleate-related substances through high performance liquid chromatography. According to the method, an afatinib dimaleate raw material drug or preparation is prepared into a detection solution with diluents, octadecyl silane keys and silica gel serve as fixing phases, inorganic salt buffer liquid is adopted as a flowing phase A, an organic phase serves as a flowing phase B, gradient elution is conducted, and an HPLC spectrogram is recorded under afatinib absorption wave length. According to the method, a chromatographic column with the octadecyl silane keys and silica gel serving as filler is adopted, the inorganic salt buffering liquid-organic phase for regulating the proper pH value serves as the flowing phase, the contents of related substances existing possibly of the afatinib dimaleate and a preparation thereof can be measured quantitatively, main impurities and various impurities can be completely separated, the peak pattern is good, regulations of Chinese pharmacopoeia are satisfied, the quality of the afatinib dimaleate and a preparation product containing the afatinib dimaleate is effectively controlled, and the method is high in specificity, high in sensitivity and good in accuracy.
Owner:BEIJING COLLAB PHARMA
Method for detecting Afatinib and relevant substances thereof
InactiveCN105424842AEfficient separationAccurate separationComponent separationTrifluoroacetic acidColumn temperature
The invention relates to a method for detecting Afatinib, relevant substances thereof, and especially cis-isomers thereof. The method comprises the following steps: carrying out gradient elution by using a high performance liquid chromatograph, wherein Phenomenex C18 (250mm*4.6mm, 5[mu]m) is adopted as a chromatographic column, an aqueous solution containing 0.2% of formic acid and 0.1% of trifluoroacetic acid is adopted as a mobile phase A, acetonitrile is adopted as a mobile phase B, the detection wavelength is 255-265nm, and the column temperature is 35-45DEG C; and carrying out gradient elution under certain conditions. The method can effectively separate and detect Afatinib, relevant substances thereof, and especially cis-isomers thereof.
Owner:HEBEI SHINEWAY PHARMA
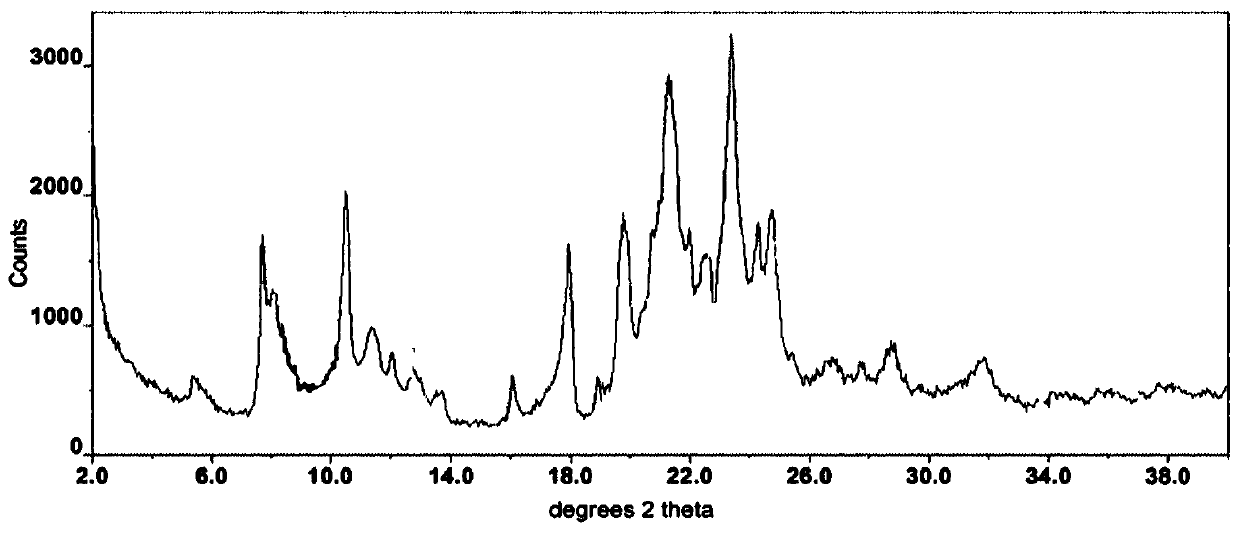
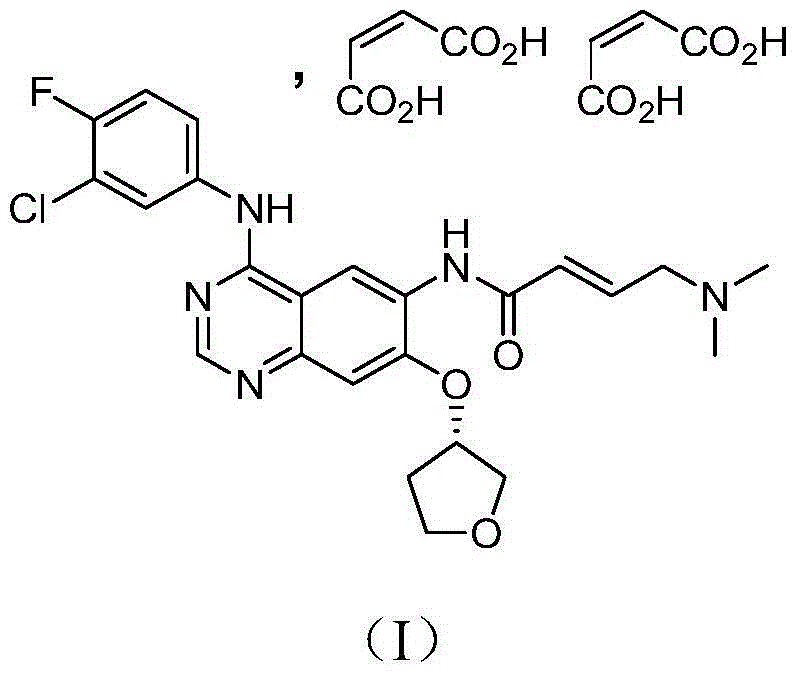
Crystal form of afatinib di-meleate and method for preparing crystal form
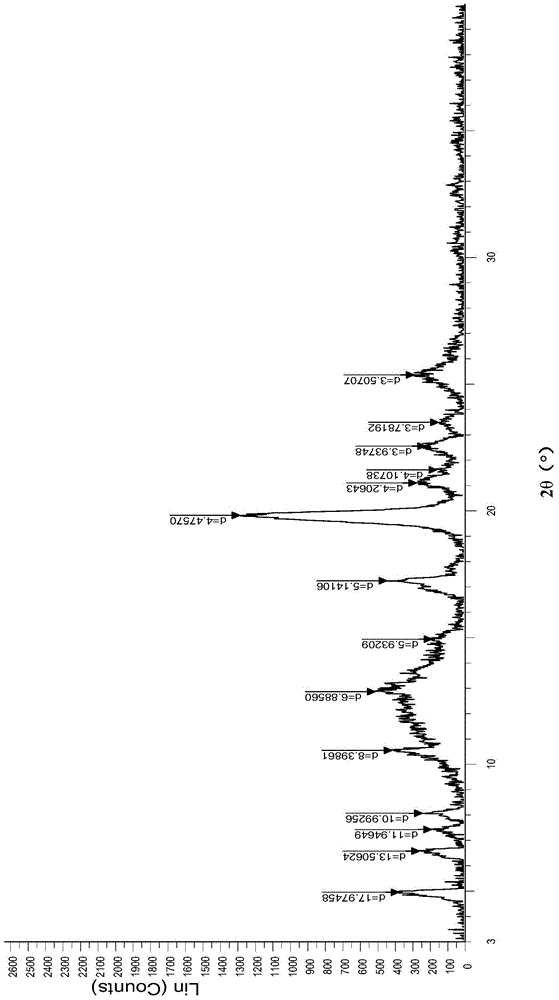
InactiveCN104926800AExcellent chemical purityImprove liquidityOrganic active ingredientsOrganic chemistryX-rayPowder diffraction
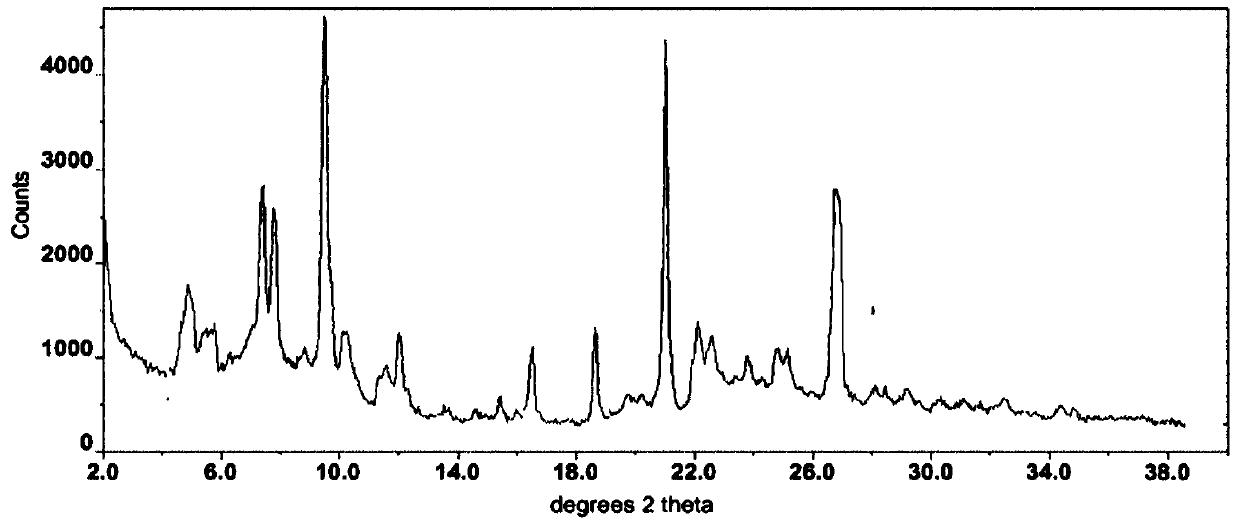
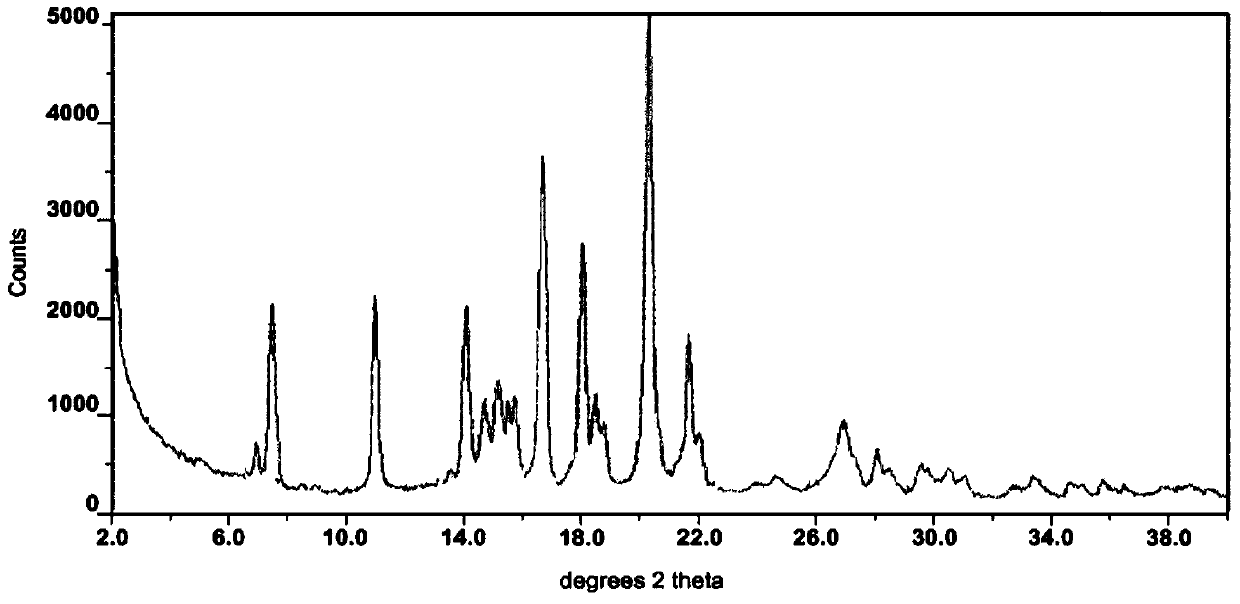
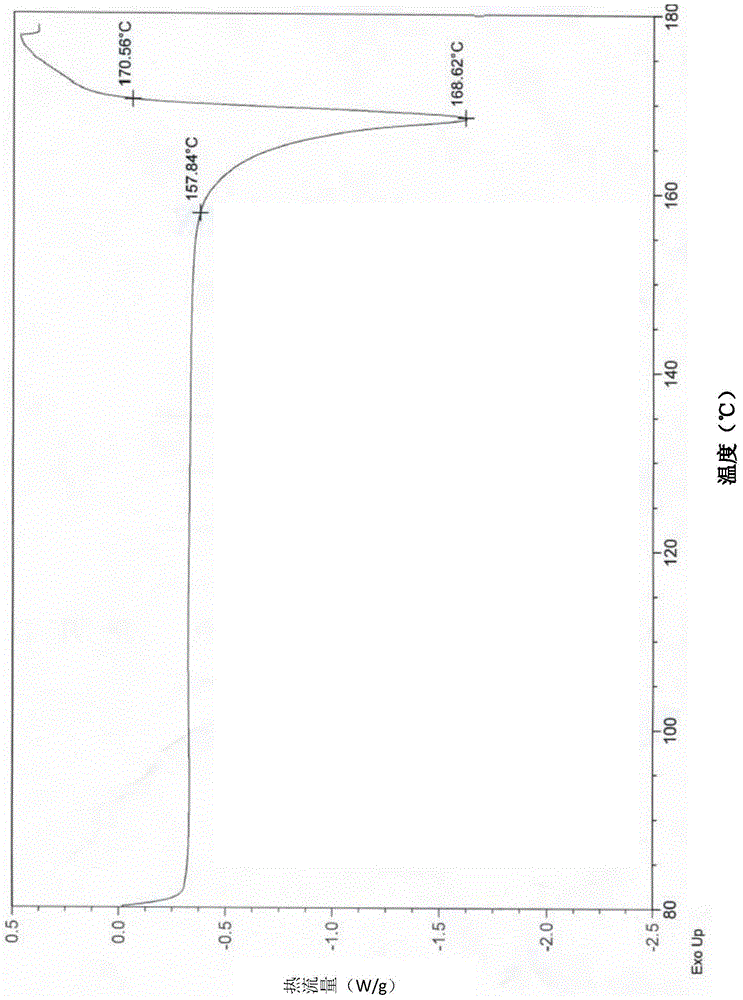
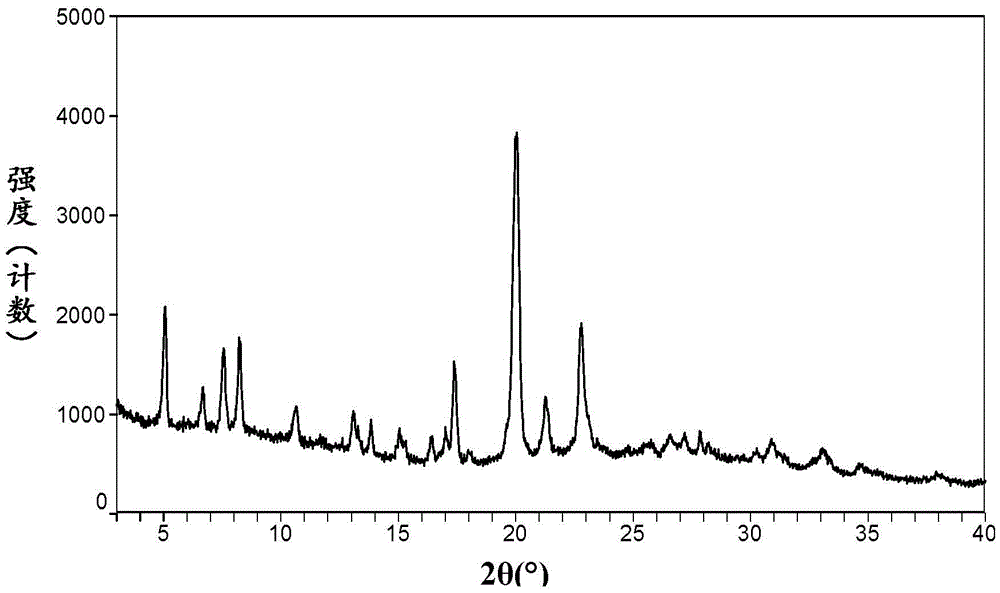
The invention relates to a crystal form of afatinib di-meleate, a method for preparing the crystal form, pharmaceutical composition with the same and application of the pharmaceutical composition. X-ray powder diffraction spectra of the crystal form have characteristic peaks at diffraction angles 2theta of 4.9+ / -0.2 degrees, 10.5+ / -0.2 degrees, 12.8+ / -0.2 degrees, 17.2+ / -0.2 degrees and 19.8+ / -0.2 degrees, and a melting point of the crystal form is 168.68. The crystal form, the method, the pharmaceutical composition and the application have the advantage that the pharmaceutical composition can be applied to anti-cancer medicine.
Owner:HEBEI SHINEWAY PHARMA
Method for preparing Afatinib
ActiveCN103254183AEase of industrial productionPromote the development of economy and technologyOrganic chemistryN dimethylformamideAniline
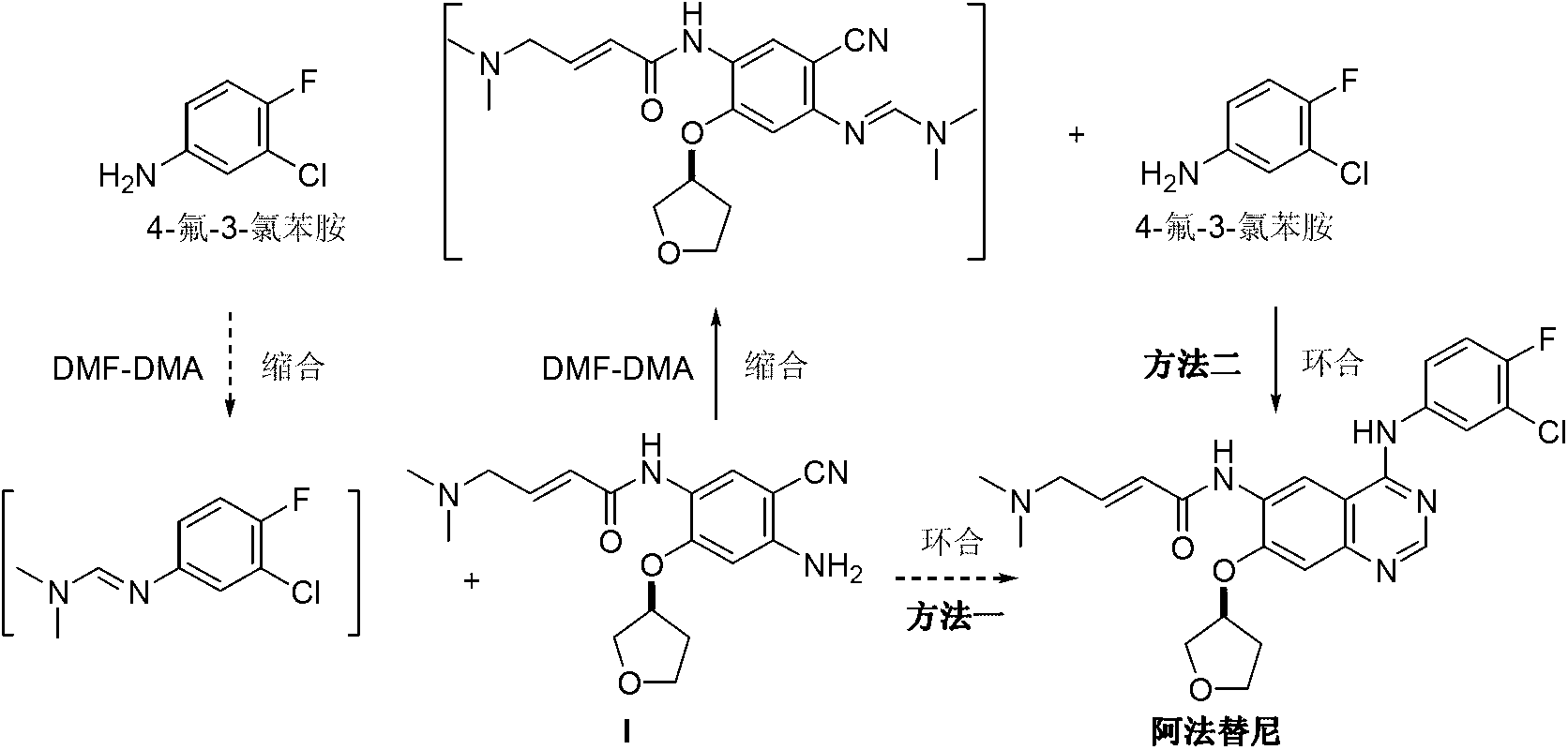
The invention discloses a method for preparing Afatinib (I). The method comprises the following steps: 2-cyano-4-[4-(N,N-dimethylamino)-1-oxo-2-buten-1-yl]amino-5-[(S)-(tetrahydrofuran-3-yl)oxy]aniline (II) and N,N-dimethylformamide dimethylacetal (DMF-DMA) are subjected to condensation reaction so as to produce an intermediate (III), and the intermediate (III), without to the need of separation, is directly subjected to cyclization reaction with 4-fluoro-3-chloroaniline so as to prepare Afatinib (I). According to the method, the steps for preparing Afatinib are reduced obviously, and the cost is reduced greatly.
Owner:铜陵尚东高新科创有限公司
Method for preparing Afatinib
InactiveCN103254182AEase of industrial productionPromote the development of economy and technologyOrganic chemistryN dimethylformamideAniline
The invention discloses a method for preparing Afatinib. The method comprises the following steps: 4-fluoro-3-chloroaniline and N,N-dimethylformamide dimethylacetal (DMF-DMA) are subjected to condensation reaction so as to produce a Schiff base (III), and the Schiff base (III), without to the need of separation, is subjected to cyclization reaction with 2-cyano-4-[4-(N,N-dimethylamino)-1-oxo-2-buten-1-yl]amino-5-[(S)-(tetrahydrofuran-3-yl)oxy]aniline so as to prepare Afatinib. According to the method, the steps for preparing Afatinib are reduced obviously, and the cost is reduced greatly.
Owner:SUZHOU MIRACPHARMA TECH +1
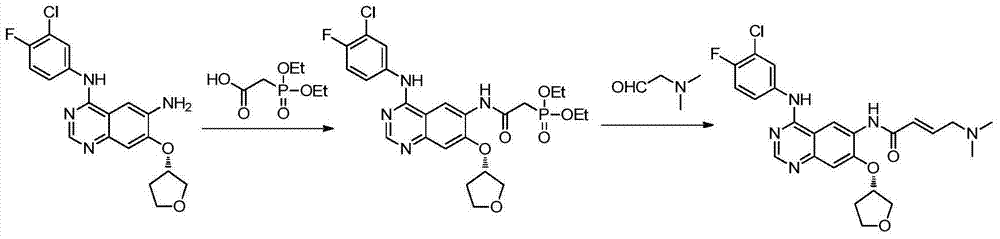
Preparation method of afatinib intermediate
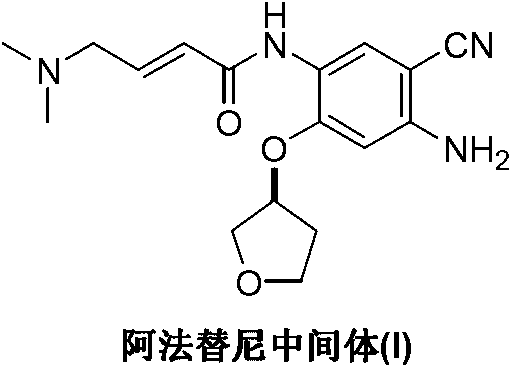
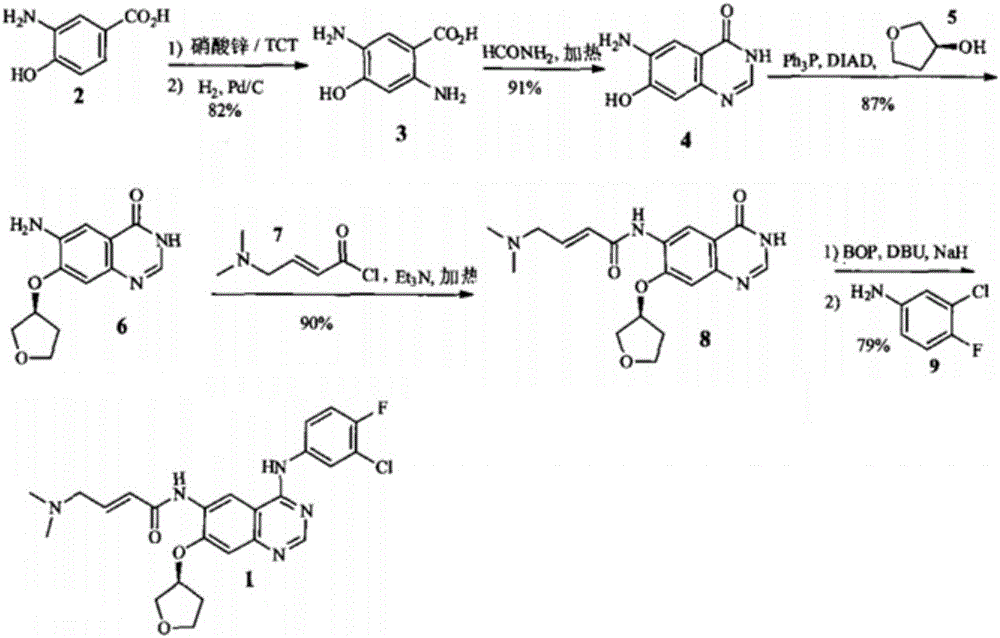
The invention discloses a preparation method of an afatinib intermediate. The preparation method comprises the following steps of: sequentially carrying out nitrification, etherification, reduction, amidation, nitrification and reduction on 4-cyanol phenol serving as the starting raw material to prepare 2-cyano-4-[4-(N,N-dimethylamino)-1-oxo-2-butene-1-yl]amino-5-[(S)-tetrahydrofuran-3-yl]oxy]phenylamine (I). The preparation method has the advantages that the process is stable, raw materials are easily available, cost is low and all reactions are classic reactions; therefore, the preparation method meets the requirement of industrial amplification.
Owner:鄄城县人民医院
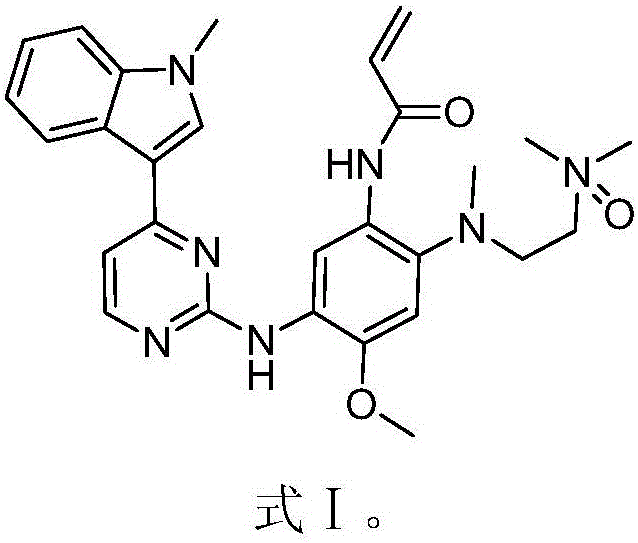
EGFR inhibitor for targetedly treating cancer and preparation method and application thereof
ActiveCN105777716ALittle side effectsOrganic chemistryAntineoplastic agentsSide effectDouble mutation
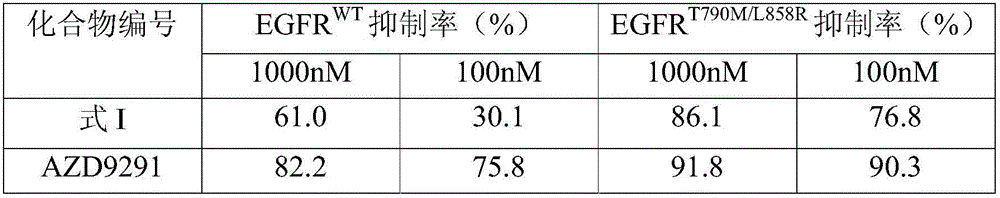
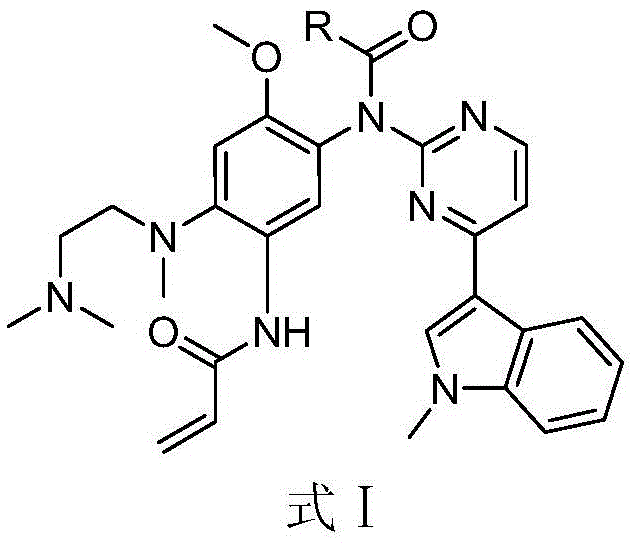
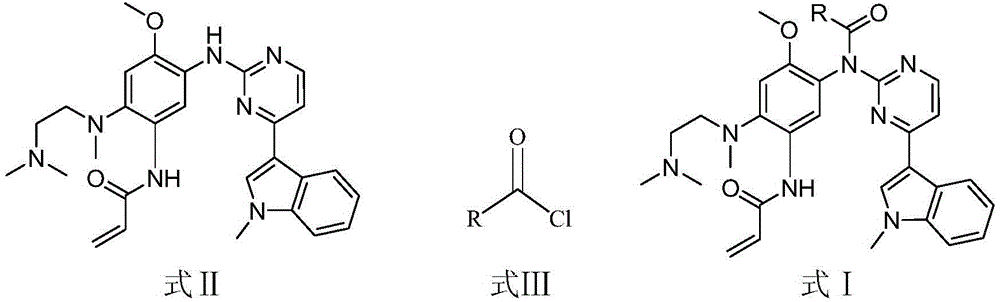
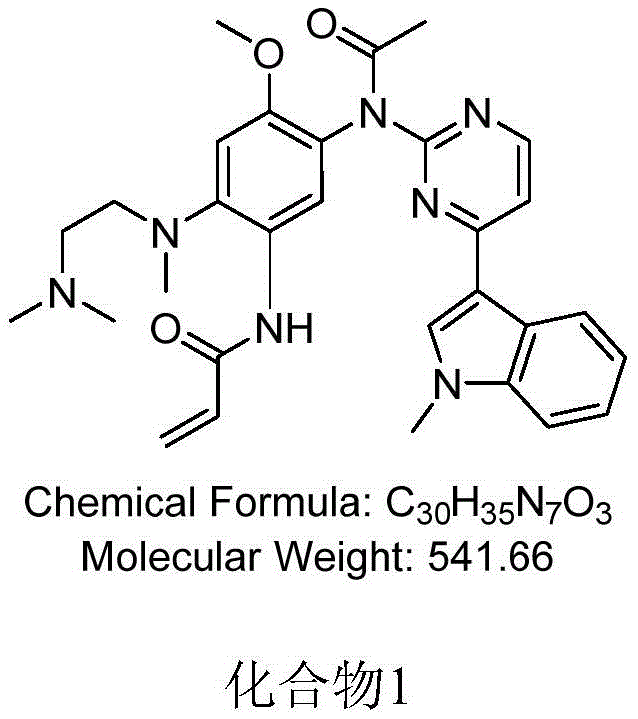
The invention discloses an EGFR inhibitor for targetedly treating a cancer and a preparation method and application thereof.The structural formula of the EGFR inhibitor is shown in the formula I.The EGFR inhibitor can be used for preventing and / or treating the cancer such as a human skin caner or a lung cancer.The EGFR inhibitor can selectively inhibit cell lines of EGFR double mutation (EGFRT790M and L858R), and the inhibitory activity to EGFR wild type cells is weak; therefore, the EGFR inhibitor can be used for treating lung cancer patients with EGFRT790M and L858R mutation, and the side effects are fewer, wherein the side effects are caused by inhibiting wild type EGFR, for example, Afatinib.
Owner:TSINGHUA UNIV
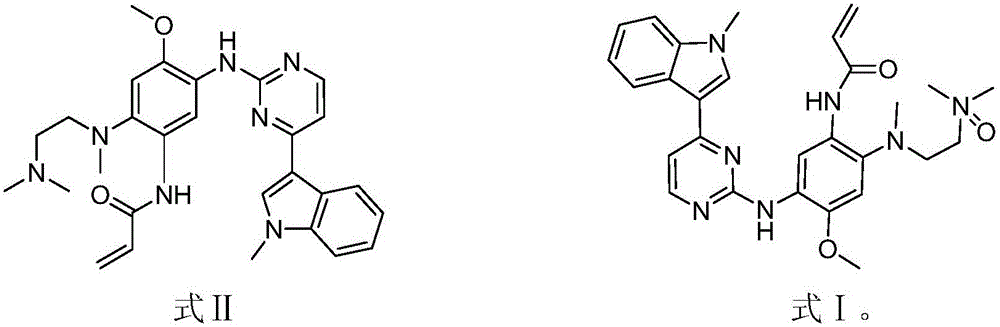
EGFR (epidermal growth factor receptor) inhibitor for targeted therapy of cancers, and preparation method and application thereof
ActiveCN105585557ANovel chemical structureLittle side effectsOrganic active ingredientsOrganic chemistryChemical structureSide effect
The invention discloses an EGFR (epidermal growth factor receptor) inhibitor for targeted therapy of cancers, and a preparation method and application thereof. The structural formula of the EGFR inhibitor is disclosed as Formula I, wherein R is H, OH, NR', C1-C3 alkyl, C1-C3 alkenyl, aryl or heterocycle, and R' is C1-C3 alkyl. The EGFR inhibitor can be used for preventing and / or treating cancers, such as human skin squamous carcinoma or lung cancer. Compared with the existing inhibitors (such as AZD9291, afatinib and the like), the EGFR inhibitor disclosed by the invention has novel chemical structure. The EGFR inhibitor can selectively inhibit cell lines of EGFR double mutants (EGFRT790M / L858R), and has lower inhibition activities for EGFR wild type cells. Therefore, the EGFR inhibitor disclosed by the invention can be used for treating the patient with lung cancer with EGFRT790M / L858R mutants, and has lower side effect (caused by the inhibition of the wild type EGFR, such as afatinib).
Owner:TSINGHUA UNIV
High-purity afatinib preparation method
The invention discloses a high-purity afatinib preparation method, which comprises: (1) adding a trans-4-dimethylaminocrotonic acid hydrochloride to the mixed solvent of N-methylpyrrolidone and ethyl acetate, cooling, adding thionyl chloride to the system in a dropwise manner, and stirring until the reaction is completely performed; (2) dissolving N4-(3-chloro-4-fluoro-phenyl)-7-(S)-tetrahydrofuran-3-yloxy)quinazoline-4,6-diamine by using N-methylpyrrolidone, adding the obtained solution to the reaction system obtained in the step (1), and stirring until the reaction is completely performed; and (3) adding purified water to the reaction system obtained in the step (2), quenching the reaction, adjusting the pH value with a sodium hydroxide solution, extracting with ethyl acetate, carrying out pressure reducing concentration, carrying out cooling crystallization, carrying out suction filtration, and drying to obtain the high-purity afatinib product. According to the present invention, the post-treatment of the preparation method is simple and convenient, the high purity product can be obtained without the additional refining, and the preparation method is suitable for industrial large-scale production.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Application of curcumin and afatinib for combined treatment of non-small cell lung cancer
ActiveCN105476996ALower doseLittle side effectsKetone active ingredientsAntineoplastic agentsCombined treatmentOncology
Owner:SHINEWAY PHARMA GRP LTD
Afatinib-maleate crystal form, and preparation method and pharmaceutical compositions thereof
ActiveCN105801568AHigh dissolution rateEasy to processOrganic active ingredientsOrganic chemistry methodsMedicineAdvanced breast
The invention relates to a novel Afatinib-maleate crystal form and a preparation method thereof. Compared with a known Afatinib salt crystal form, the Afatinib-maleate crystal form provided by the invention has a plurality of improved characteristics. The invention also relates to pharmaceutical compositions containing the Afatinib-maleate crystal form, and application in preparation of drugs used for treatment of advanced non-small cell lung cancer (NSCLC) and HER2 positive advanced breast cancer.
Owner:倪云
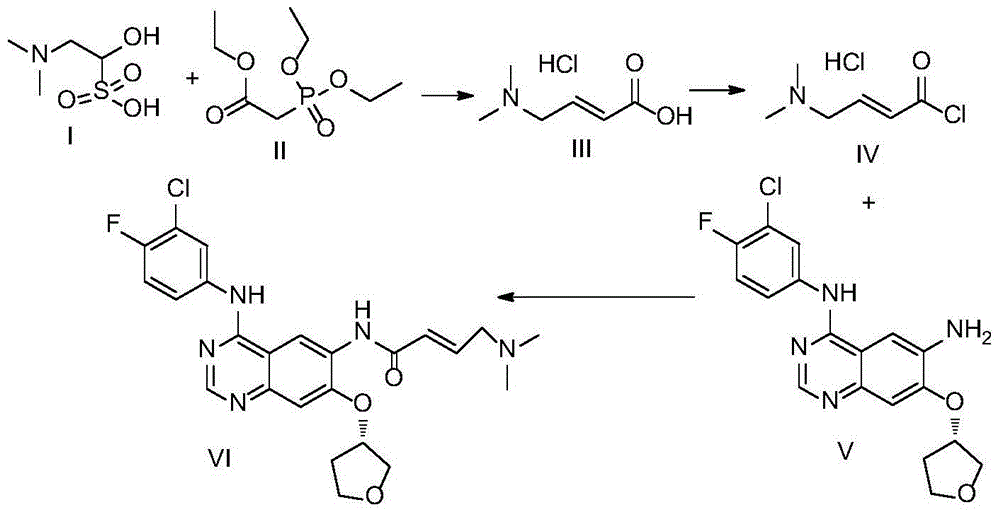
Preparation method for afatinib
ActiveCN105330652AHigh purityHigh yieldOrganic compound preparationAmino-carboxyl compound preparationChlorideCombinatorial chemistry
The invention provides a preparation method for afatinib. The preparation method comprises the following steps: subjecting trans-4-dimethylaminocrotonic acid hydrochloride (III) to chlorination so as to obtain trans-4-dimethylaminocrotonyl chloride hydrochloride (IV); and reacting the compound (IV) with N4-(3-chloro-4-fluoro-phenyl)-7-((S)-tetrahydrofuran-3-yl-oxy)quinazoline-4,6-diamine (V) so as to prepare afatinib (VI). Reaction equations are described in the specification.
Owner:TIANJIN PHARMACN MEDICAL TECH
Autophagy inhibitor and afatinib medicine composition and application of composition to preparation of tumor synergist
InactiveCN109419803AEnhance the ability to kill tumor cellsReduce dosageAntineoplastic agentsHeterocyclic compound active ingredientsSide effectTumor cell apoptosis
The invention belongs to the technical field of medicines, and relates to an autophagy inhibitor and afatinib medicine composition and an application of the composition to preparation of a tumor synergist. According to a combination medicine, afatinib remarkably inhibits proliferation of tumor cells and induces apoptosis and autophagy of tumor cells, autophagy protects the tumor cells when the tumor cells are killed and wounded by the afatinib, so that autophagy inhabitation is obviously enhanced, and the killing and wounding effects of the afatinib on the tumor cells are improved. According to the composition, treatment dosage of the afatinib, toxic and side effects generated by medicines and medicine resistance can be reduced, and treatment cost is reduced. The composition is used for intervention treatment of chronic myelogenous leukemia, liver cancer, breast cancer, non-small cell lung cancer and tumors caused by EGFR (epidermal growth factor receptor) and HER2 (human epidermal growth factor receptor 2) mutation in a united or sequential mode.
Owner:FUDAN UNIV
Afatinib-Containing Formulation
ActiveUS20190029962A1Improve stabilityImproved profilePowder deliveryOrganic active ingredientsSolubilityOrganic solvent
The present invention refers to a process for preparing granules or particles comprising afatinib dimaleate, comprising the steps of providing afatinib dimaleate and at least one pharmaceutically acceptable excipient, and preparing granules or particles involving the use of at least one solvent selected from organic solvent and water, wherein all pharmaceutically acceptable excipients used in the process for preparing granules or particles have neutral or acidic properties, and wherein afatinib dimaleate has a solubility of at least 5 mg / ml in said at least one solvent. The present invention further refers to granules or particles comprising afatinib dimaleate that are prepared according to this process. Additionally, the present invention refers to a process for preparing a pharmaceutical composition comprising afatinib dimaleate, as well as to an adsorbate comprising afatinib dimaleate. Finally, the present invention refers to a pharmaceutical composition comprising afatinib dimaleate for use in a method of treating certain diseases.
Owner:SANDOZ AG
Compounds with Anti-tumor activity against cancer cells bearing EGFR or her2 exon 20 mutations
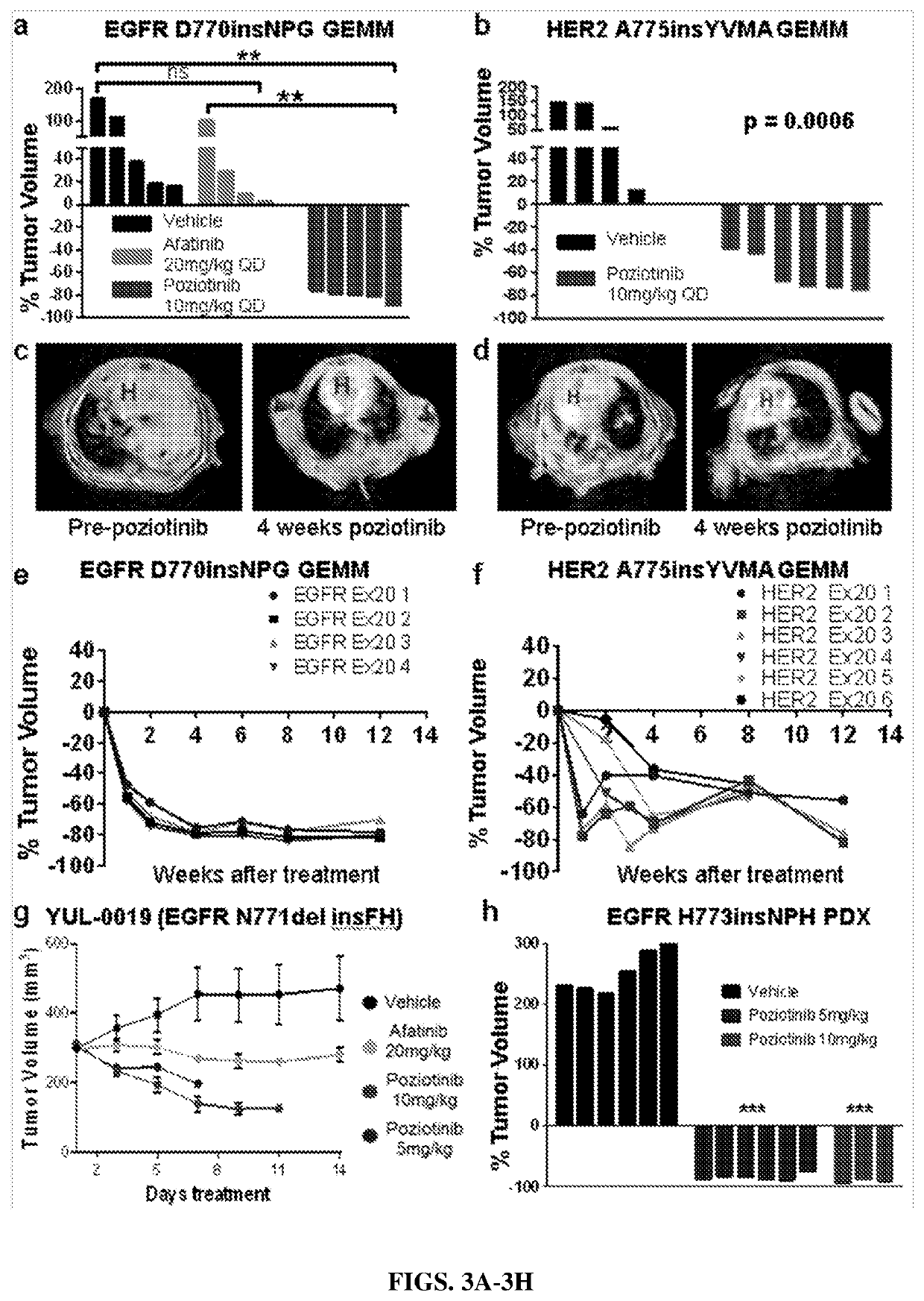
ActiveUS20200316071A1Improve responseReduce the burden onOrganic active ingredientsMicrobiological testing/measurementCancer cellTyrosine-kinase inhibitor
Owner:BOARD OF REGENTS
Compound, and preparation method and application thereof
InactiveCN106916147AEfficient preparationStarting materials are cheap and readily availableOrganic chemistryHydrogenOrganic solvent
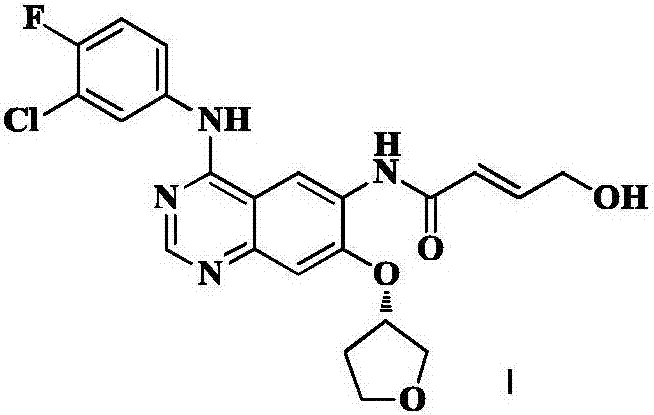
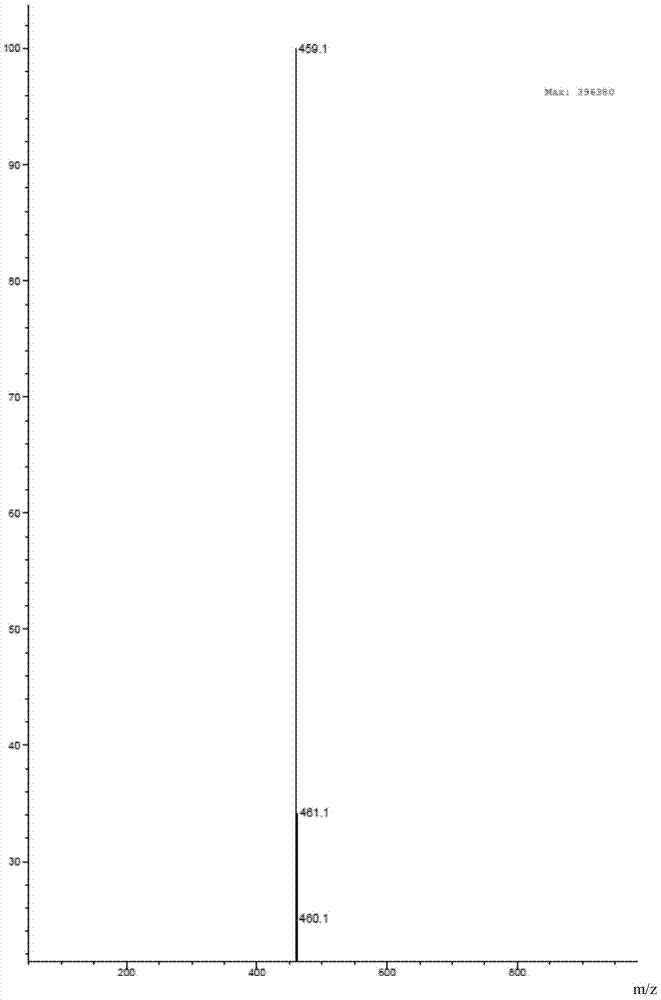
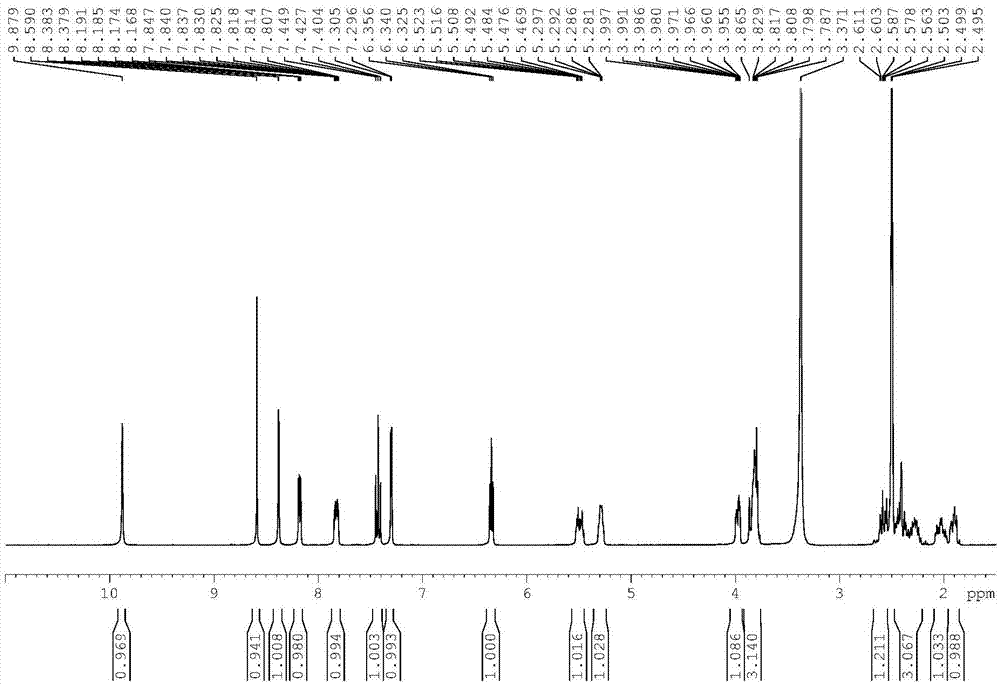
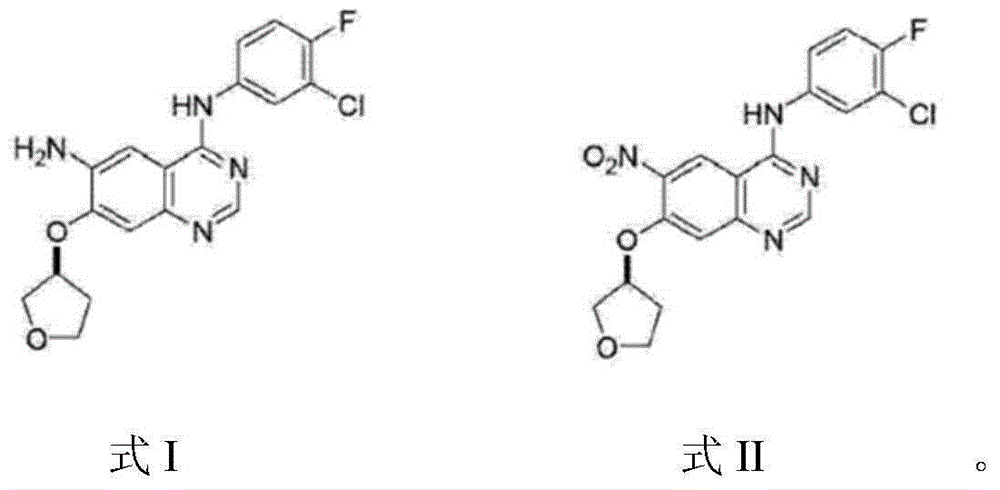
The invention relates to a compound, and a preparation method and application thereof. Specifically, the compound is as shown in a formula 1 which is described in the specification. The invention also provides the preparation method for the compound as shown in the formula 1. The preparation method comprises a step of contacting N-[4-[(3-chloro-4-fluorophenyl)amino]-7[[(3S)-tetrahydro-3-furyl]oxy]-6-quinazolinyl]-4-(dimethylamino)-2-butyleneamide with an alkaline aqueous solution in an organic solvent so as to obtain the compound as shown in the formula 1. The preparation method is simple to operate; a white powder product can be obtained through direct filtering after post-treatment; and the prepared compound has high purity, as high as 99% or above, and can be directly used as an impurity control substance for research on the quality of an afatinib bulk drug.
Owner:WATERSTONE PHARMA WUHAN
Afatinib preparation method
ActiveCN103242303BEase of industrial productionPromote the development of economy and technologyOrganic chemistry3-Hydroxytetrahydrofuran2-Butene
The invention discloses an Afatinib (I) preparation method which comprises the following steps: performing etherification reaction on 4-chloro-6-amino-7-hydroxyquinazoline (II) and (S)-3-hydroxytetrahydrofuran to generate 4-chloro-6-amino-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline (III); performing acylation reaction on the compound (III) and 4-(N,N-dimethylamino)-2-ene-butyryl chloride to generate 4-chloro-6-{[4-(N,N-dimethylamino)-1-oxo-2-butene-1-yl]amino}-7-[(S)-(tetrahydrofuran-3-yl)oxy]quinazoline (IV); and performing condensation reaction on the compound (IV) and 4-fluoro-3-chloroaniline to obtain Afatinib (I). The preparation method is simple, economic and environment-friendly in process, and meets the requirements for large-scale industrialization.
Owner:铜陵尚东高新科创有限公司
Composition containing protein kinase inhibitor and metformin
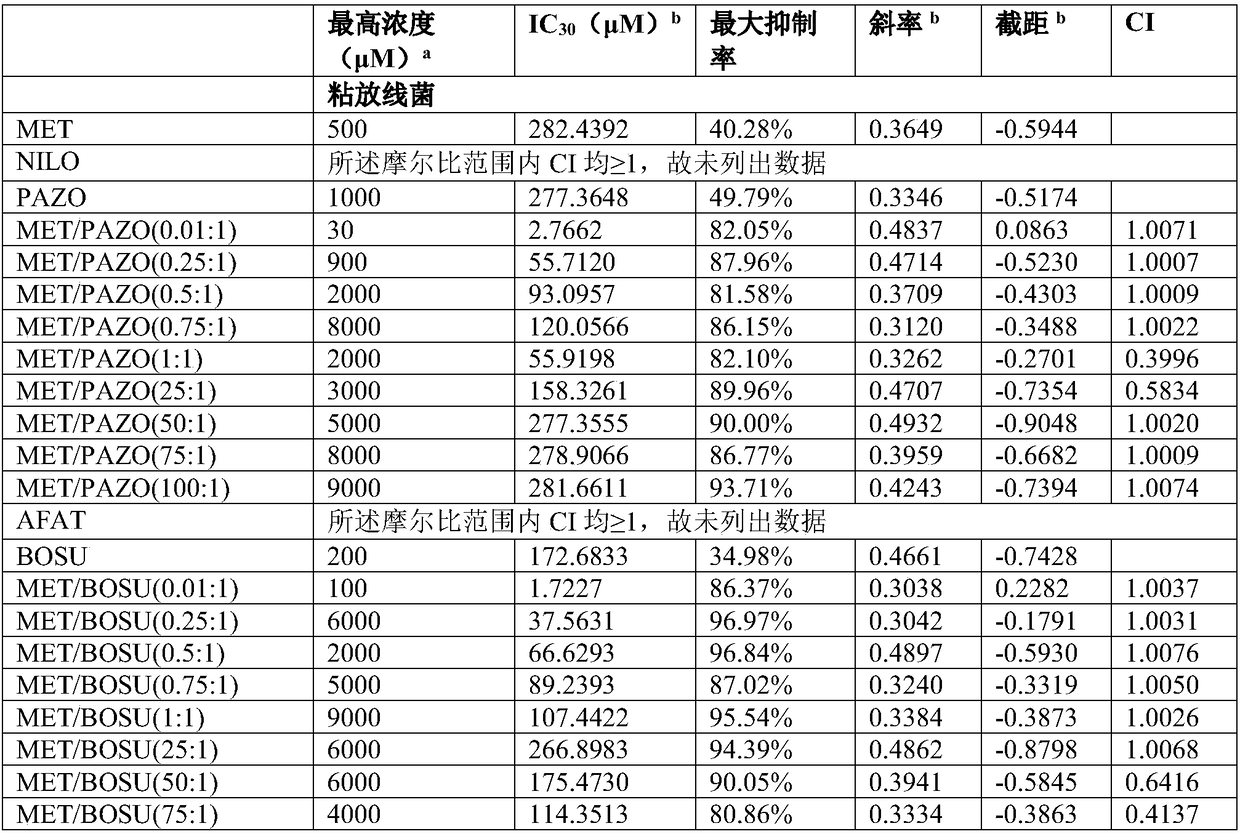
The invention firstly provides a composition containing a protein kinase inhibitor and metformin or pharmaceutically acceptable salts thereof. The composition is characterized in that the protein kinase inhibitor is one selected from nilotinib, pazopanib, afatinib, bosutinib, crizotinib, axitinib and regorafenib or pharmaceutically acceptable salts or solvates thereof or solvates of the pharmaceutically acceptable salts thereof, and the molar ratio of the metformin to the protein kinase inhibitor is (0.01-100):1. In-vitro bacteriostatic tests find that the composition containing the metforminand the protein kinase inhibitor can achieve a synergistic bacteriostatic effect on various bacteria such as staphylococcus aureus in the molar ratio of (0.01-100):1 (at an inhibition rate of 30%, thecombined medication index CI is smaller than 1).
Owner:黄泳华
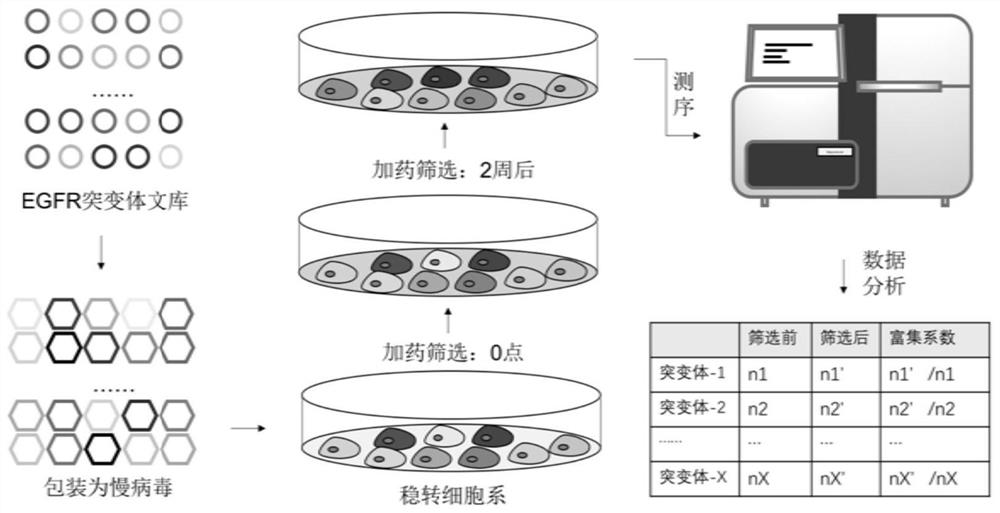
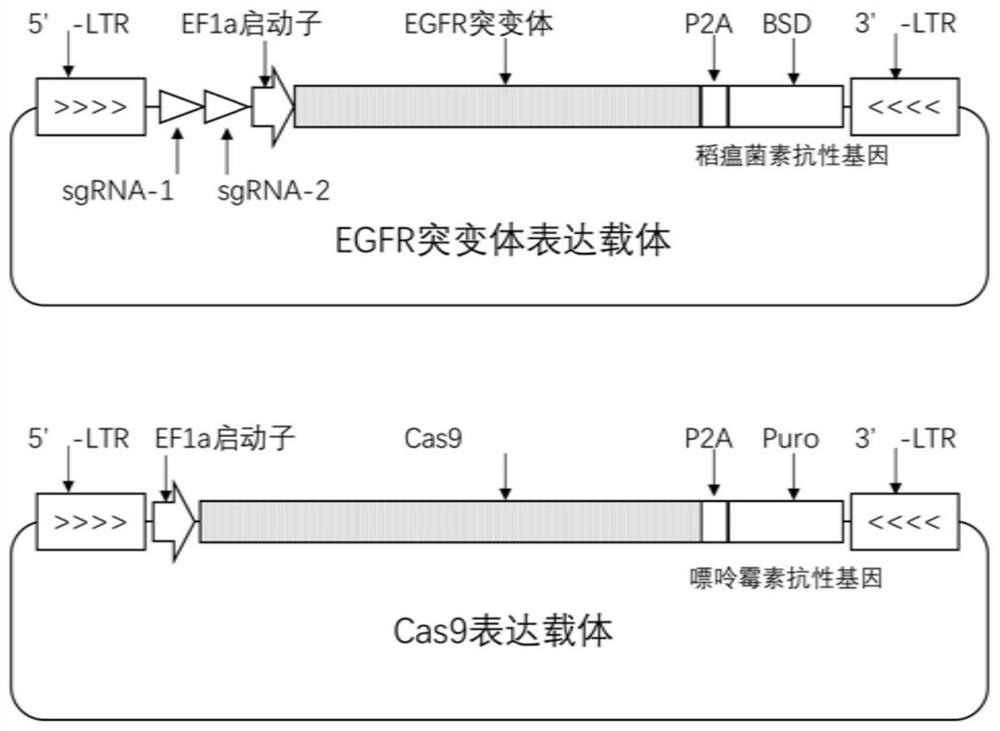
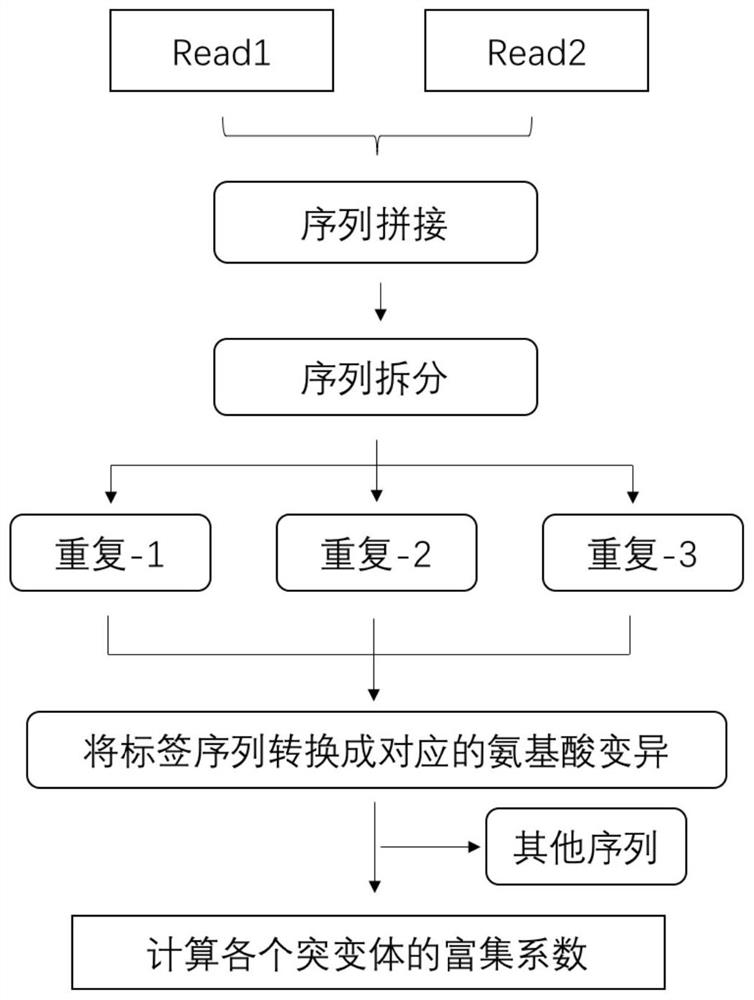
Human EGFR gene missense mutation molecular marker and application thereof in predicting drug resistance of targeted inhibitor
The invention belongs to the field of biomedicine and gene detection, and particularly relates to a human EGFR gene missense mutation molecular marker and application thereof in predicting drug resistance of a targeted inhibitor. The molecular marker provided by the invention comprises 242 types of EGFR missense mutants related to the drug resistance of erlotinib, gefitinib and icotinib, and other 15 types of EGFR missense mutants related to the drug resistance of afatinib and osimertinib, and the mutants can be used for predicting the drug resistance of non-small cell lung cancer patients to targeted inhibitor treatment. According to the invention, a mutant library of EGFR gene tyrosine kinase functional regions is constructed by using a synthetic biology method, and the EGFR mutants are highly enriched in drug screening; the molecular marker can be clinically used as a potential molecular marker for predicting drug resistance of a lung cancer patient after the lung cancer patient is treated by a targeted inhibitor.
Owner:卫国朋
Method of blocking transmission of malarial parasite
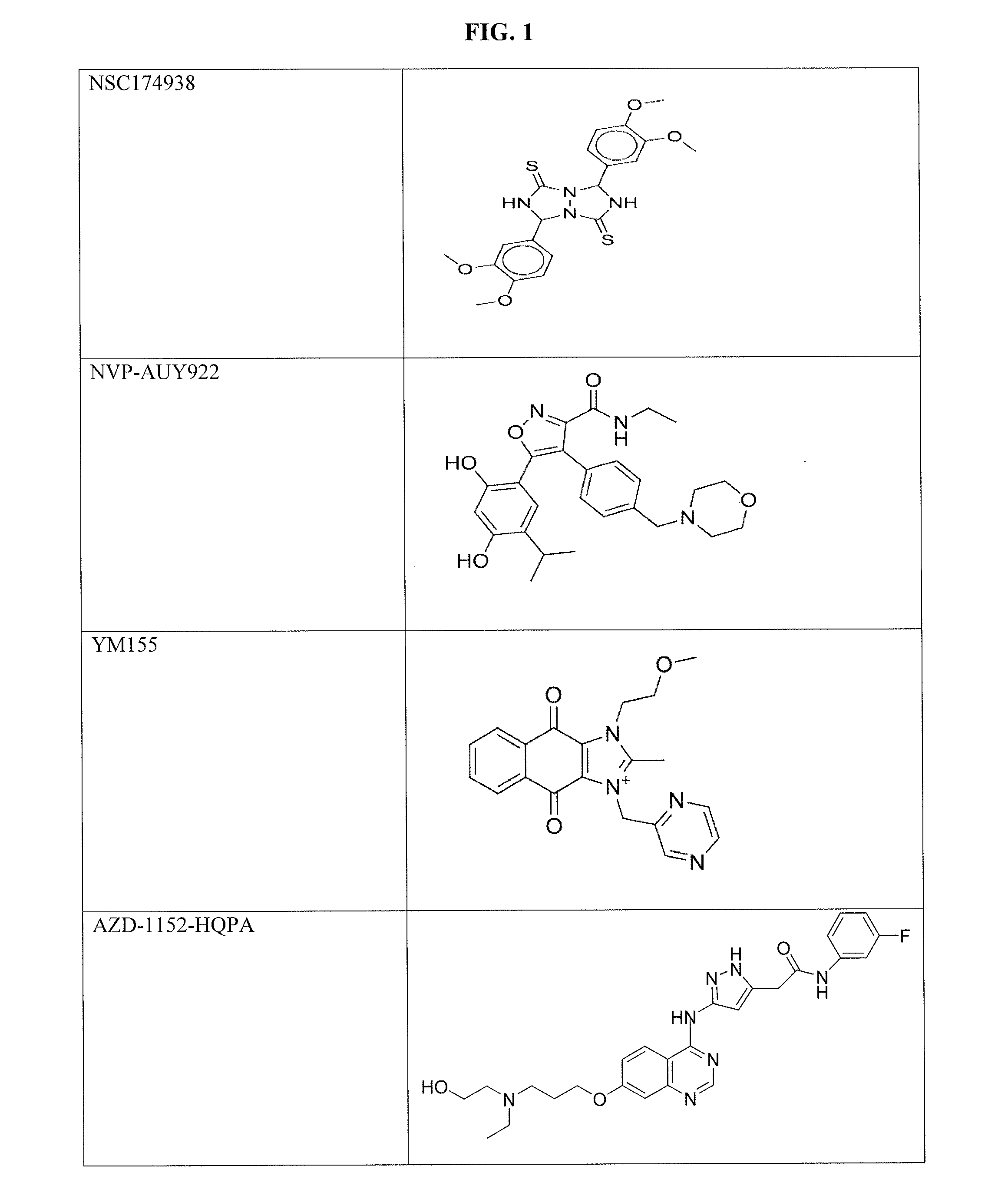
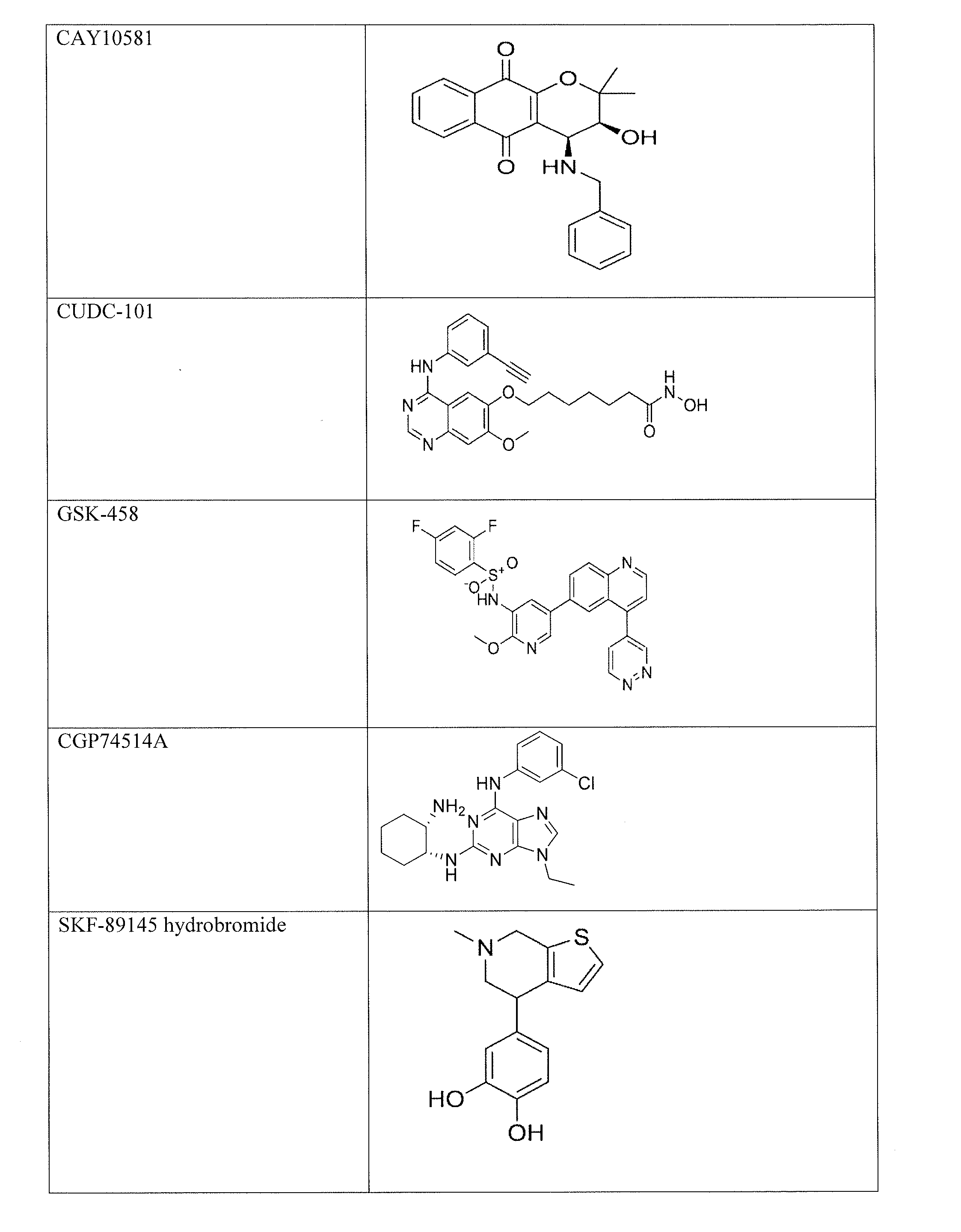
The invention provides a method of blocking transmission of a Plasmodium parasite and a method of treating or preventing malaria comprising administering to an animal an effective amount of a first compound of formula I: wherein A, B, R1, R2, R10, and R11 are described herein, either alone or in combination with a second compound selected from elesclomol, NSC 174938, NVP-AUY922, Maduramicin, Narasin, Alvespimycin, Omacetaxine, Thiram, Zinc pyrithione, Phanquinone, Bortezomib, Salinomycin sodium, Monensin sodium, Dipyrithione, Dicyclopentamethylene-thiuram disulfide, YM155, Withaferin A, Adriamycin, Romidepsin, AZD-1 152-HQPA, CAY10581, Plicamycin, CUDC-101, Auranofin, Trametinib, GSK-458, Afatinib, and Panobinostat.
Owner:UNITED STATES OF AMERICA +1
Synthesis method of anti-tumor medicine afatinib
ActiveCN105968103ASimple process routeMild reaction conditionsOrganic chemistryAntineoplastic agentsAbnormal tissue growthChemical reaction
The invention discloses a synthesis method of an anti-tumor medicine afatinib and belongs to the technical field of pharmaceutical chemistry. The method takes 2-nitryl-5-bromophenol as a raw material and obtains the afatinib through a five-step chemical reaction. The raw material of a synthesis route is easy to obtain, a process route is shortened, the operation is simple and the yield of products is high; and industrial production is easy to realize.
Owner:SHANDONG LUOXIN PHARMA GRP HENGXIN PHARMA CO LTD
Preparation method of Afatinib intermediate
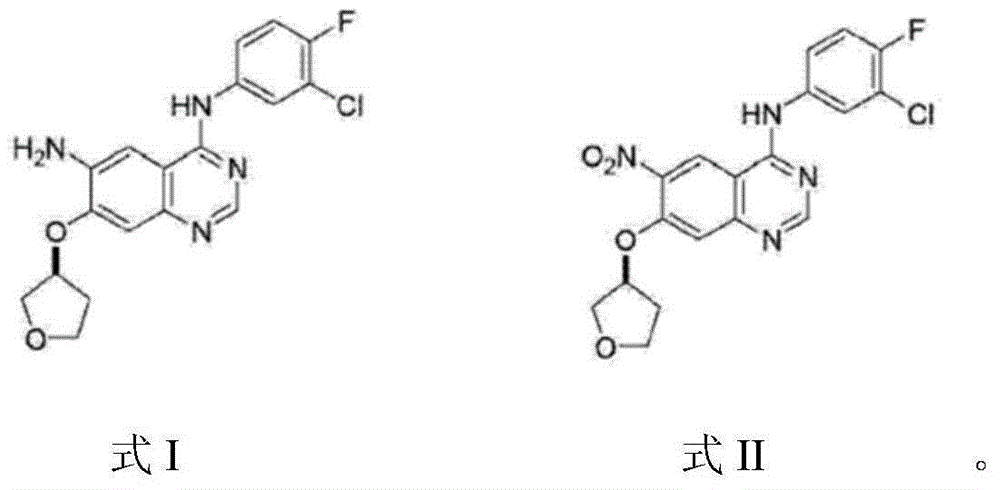
The invention provides a preparation method of an Afatinib intermediate and particularly provides a preparation method of N<4>-(3-chloro-4- fluorophenyl)-7-[[(3S)-tetrahydro-3-furyl]oxy]-4,6-quinazoline diamine. According to the method, 4-[(3-chloro-4-fluorophenyl)amino]-6-nitro-7-((S)-tetrahydrofuran-3-yloxy)-quinazoline, zinc powder and an acidic material are mixed in a mixed solvent of a polar solvent and water for a reduction reaction, a product is filtered and alkalified, and the Afatinib intermediate is obtained. The preparation method has the advantages of high product yield, low pollution degree, low production cost and the like.
Owner:SHINEWAY PHARMA GRP LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com