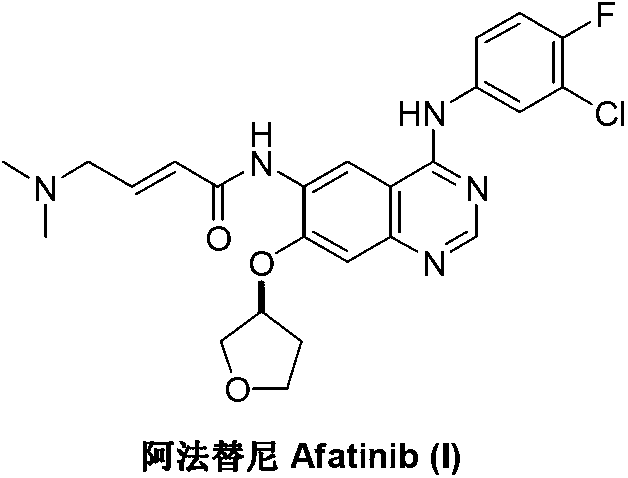
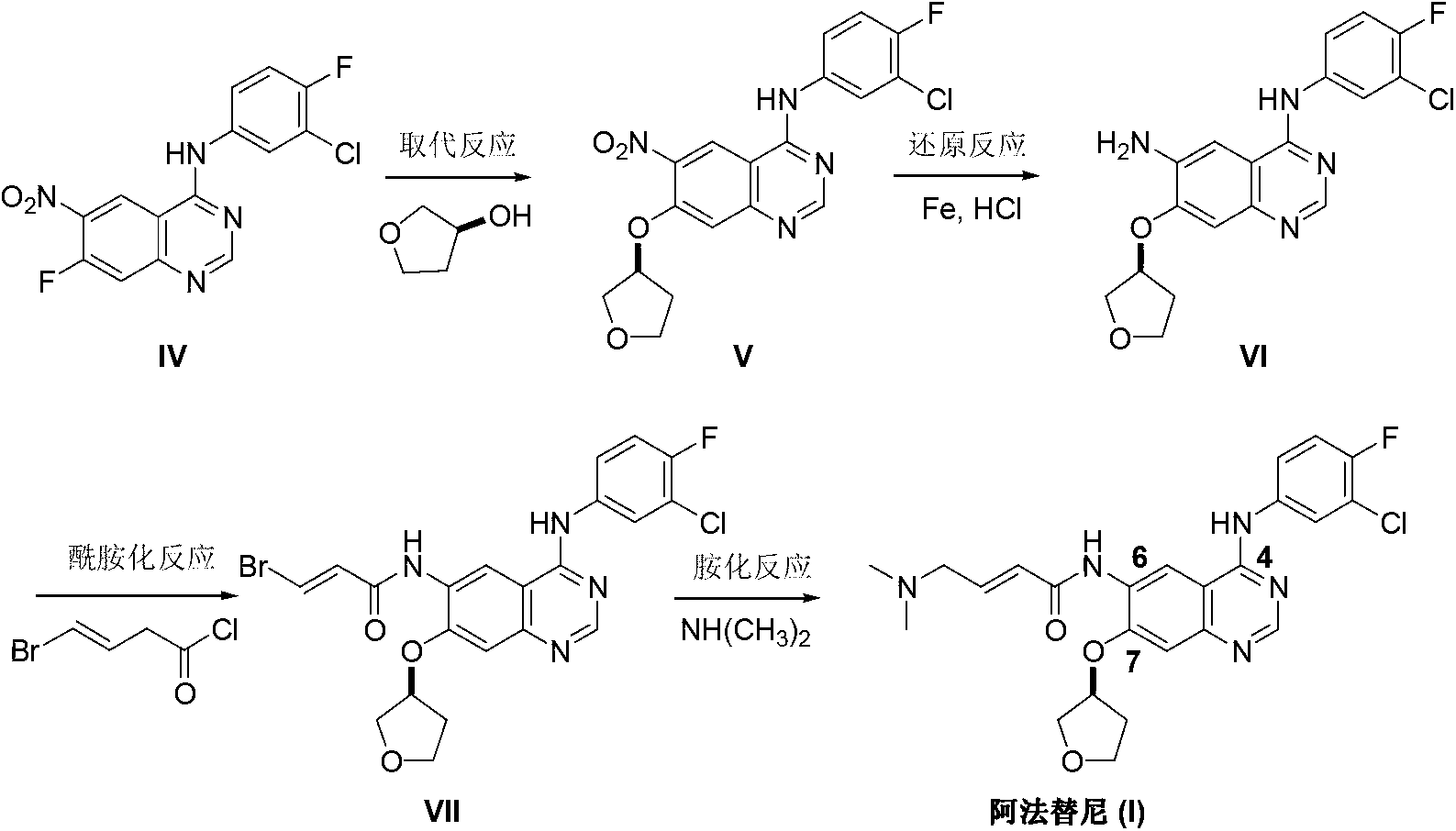
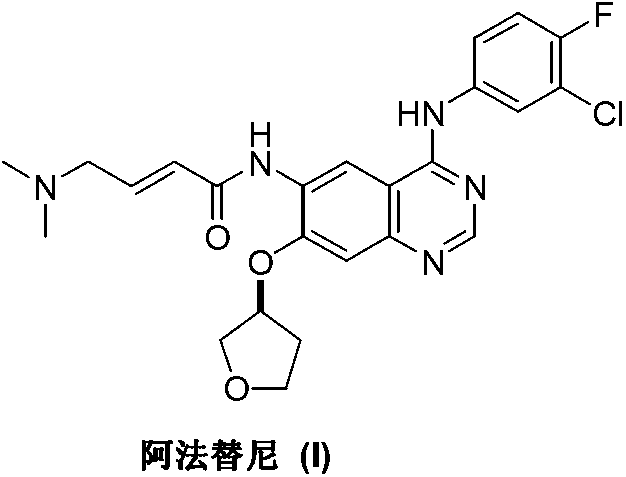
Method for preparing Afatinib
A technology of afatinib and amino, which is applied in the field of preparation of afatinib, can solve the problems of many steps, low total yield, unsuitable for industrialization, etc., and achieve the goals of easy availability of raw materials, promotion of development, and promotion of economic technology Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 2-cyano-4-[4-(N,N-dimethylamino)-1-oxo-2-buten-1-yl]amino-5-[(S)- into a 500mL three-necked flask (tetrahydrofuran-3-yl)oxy]aniline (II) (12.0 g, 0.036 mol), N,N-dimethylformamide dimethyl acetal (DMF-DMA) (6.5 g, 0.054 mol) and toluene 150mL. Add catalyst anhydrous acetic acid 3.5mL under stirring. The temperature was raised to 105-110° C., and the temperature was maintained for 3 hours to react (methanol was collected with an oil-water separator), and the reaction was monitored by TLC to complete. Toluene was recovered by distillation under reduced pressure at 50° C. to obtain 12.8 g of a light brown oil with a yield of 92.4%, which can be directly used in the following reaction without separation.
[0026] The above oil was dissolved in 150 mL of anhydrous acetic acid, and transferred to a 500 mL three-necked flask. 3-Chloro-4-fluoroaniline (7.13 g, 0.049 mol) was added. After stirring, the temperature was raised to 115-125° C. and kept under reflux for 6 hou...
Embodiment 2
[0028] Add 2-cyano-4-[4-(N,N-dimethylamino)-1-oxo-2-buten-1-yl]amino-5-[(S)- into a 500mL three-necked flask (tetrahydrofuran-3-yl)oxy]aniline (II) (12.0 g, 0.036 mol), N,N-dimethylformamide dimethyl acetal (DMF-DMA) (6.5 g, 0.054 mol) and toluene 150mL. Add catalyst anhydrous formic acid 3.4mL under stirring. The temperature was raised to 105-110° C., and the temperature was maintained for 3 hours to react (methanol was collected with an oil-water separator), and the reaction was monitored by TLC to complete. After cooling, the toluene was recovered by distillation under reduced pressure at 50° C. to obtain 12.1 g of a brown oil with a yield of 87.3%, which can be directly used in the following reaction without separation.
[0029] The above oil was dissolved with 25mL of anhydrous acetic acid and 125mL of toluene, and transferred to a 500mL three-necked flask. 3-Chloro-4-fluoroaniline (7.0 g, 0.048 mol) was added. After stirring, the temperature was raised to 120-130° C....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com