Patents
Literature
96 results about "Dimethylformamide-dimethylacetal" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
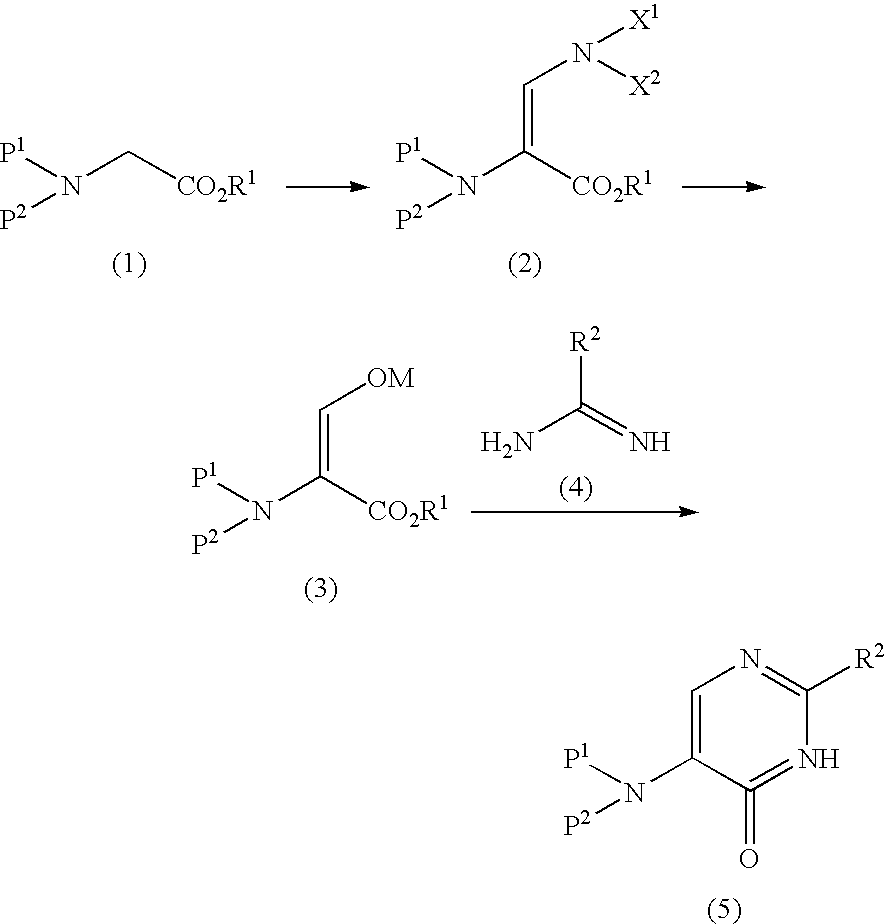
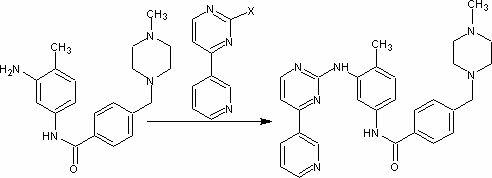
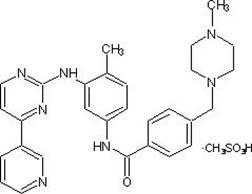
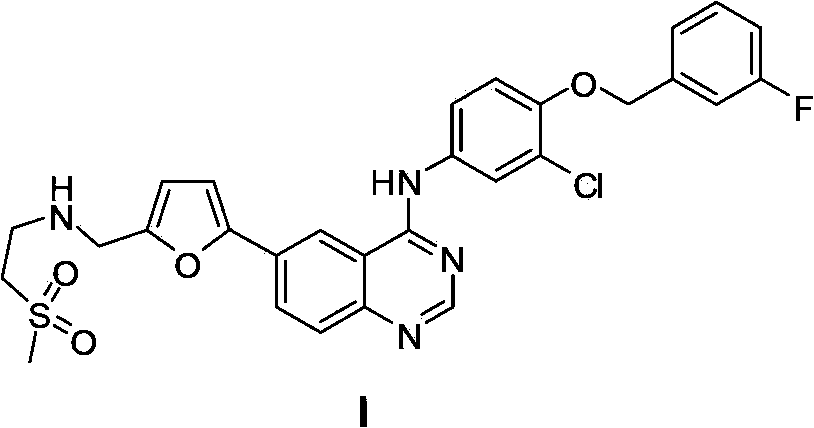
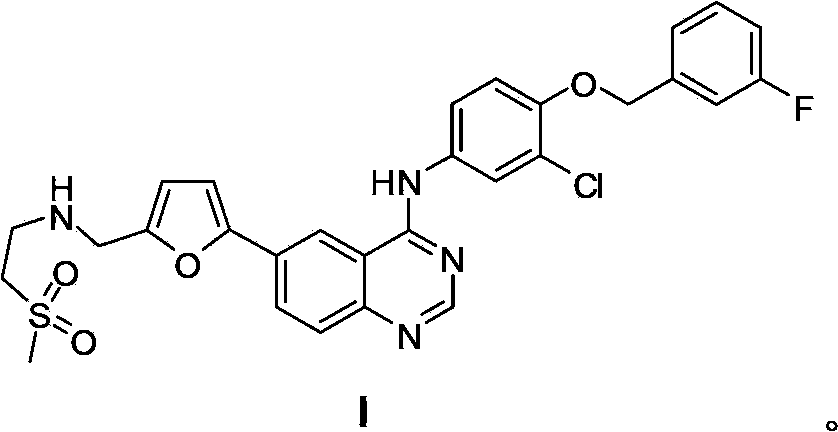
5-Protected aminopyrimidine compound, production method thereof and intermediate therefor
InactiveUS20050209257A1High yieldHigh purityBiocideOrganic active ingredientsAmidineDimethylformamide-dimethylacetal
The present invention provides a production method of 5-aminopyrimidine compound represented by the formula (5) by reacting a glycine compound represented by the formula (1) with t-butoxybisdimethylaminomethane, dimethylformamidedimethylacetal or dimethylformamidediethylacetal to produce a dialkylaminomethylene compound represented by the formula (2), reacting the compound of formula (2) in the presence of an acid to produce a hydroxymethylene compound represented by the formula (3), and reacting the compound of formula (3) with an amidine compound represented by the formula (4) or a salt thereof.
Owner:AJINOMOTO CO INC
Preparation method of imatinib intermediate
InactiveCN102603710AMild reaction conditionsSimple reaction conditionsOrganic chemistryThioureaOncology
Owner:成都格蓝洋生物医药科技有限公司
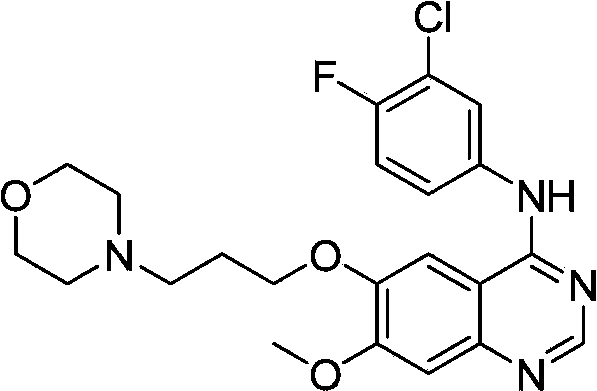
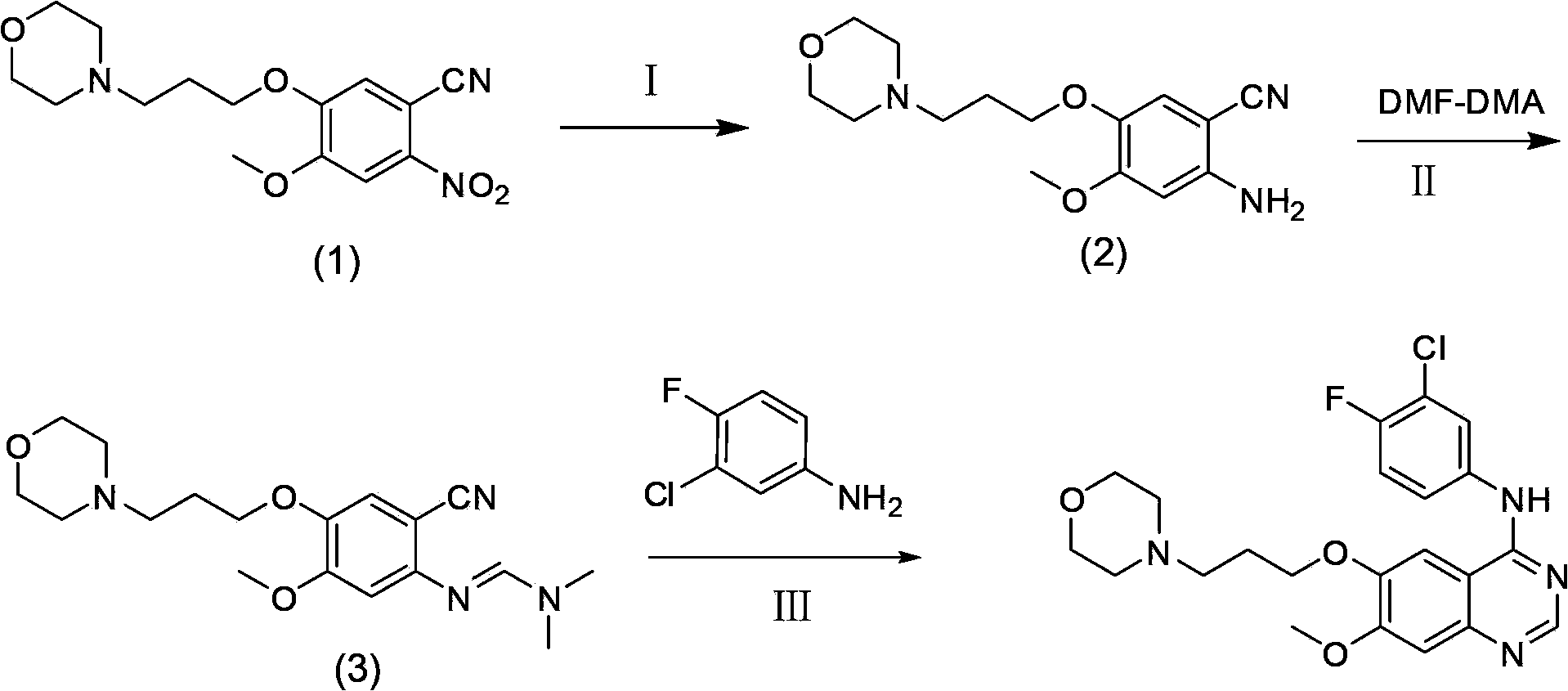
Preparation method of gefitinib
ActiveCN103570633AReduce usageFor the purpose of purificationOrganic chemistryPurification methodsMorpholine
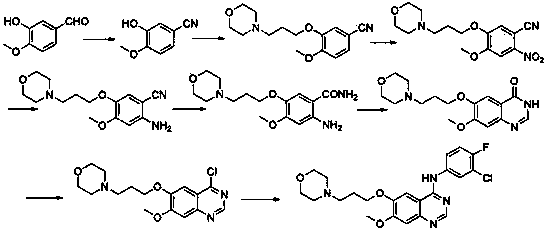
The invention discloses a preparation method of gefitinib. The preparation method takes 4-methoxyl-5-(3-morpholine propoxyl)-2-nitrobenzonitrile as a raw material, then subjecting the raw material to treatments of reduction and salt forming reactions so as to obtain an intermediate 2-amino-4-methoxyl-5-(3-morpholine propoxyl) benzonitrile hydrochloride, then directly subjecting the intermediate to react with N,N-dimethyl formamide dimethyl acetal so as to obtain N'-(2-cyano-5-methoxyl-4-(3-morpholinyl propoxyl)benzyl)-N,N-dimethyl formamidine, and finally subjecting the formamidine intermediate to carry out rearrangement reactions with 3-chloro-4-fluoroaniline so as to obtain the gefitinib. The preparation method has the advantages of mild reaction conditions, convenient intermediate purification method, and suitability for industrial production.
Owner:GUANGZHOU INST OF BIOMEDICINE & HEALTH CHINESE ACAD OF SCI
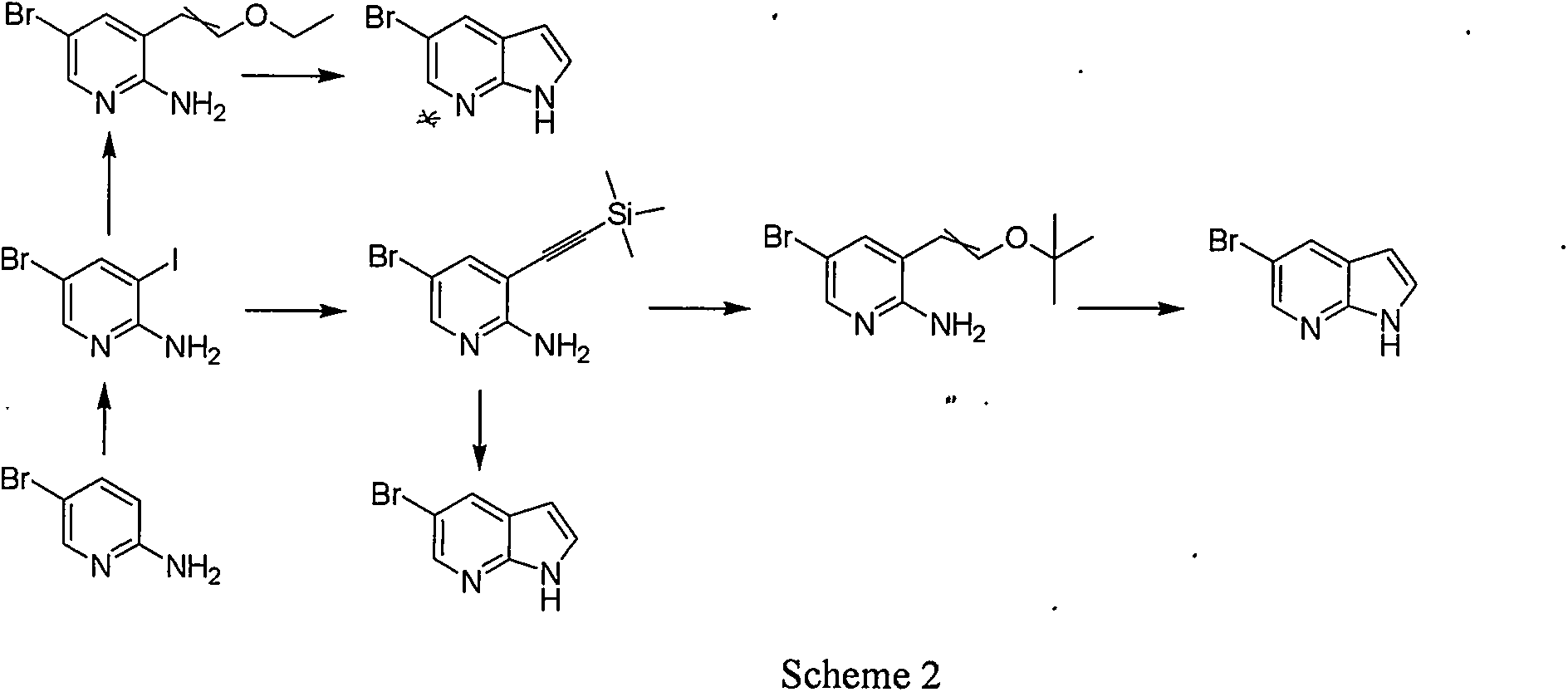
Synthetic process of 5-bromo-7-azaindole
InactiveCN104387384ARaw materials are easy to getShort reaction stepsOrganic chemistryN dimethylformamideDimethyl acetal
The invention relates to a synthetic process of 5-bromo-7-azaindole which is an important medical intermediate. The synthetic process comprises the steps of generating 2-nitro-3-methyl-5-bromopyridine from 2-amino-3-methyl-5-bromopyridine by virtue of the oxidation of caro acid, and generating an intermediate 3 under the actions of tetrahydropyrrole and N,N-dimethylformamide dimethyl acetal (DMF-DMA), and finally carrying out reduction and loop closing under the action of a raney nickel / 85% hydrazine hydrate system or other low valence metal so as to generate 5-bromo-7-azaindole. The synthetic process is suitable for industrial production and has the advantages that the raw material cost is low, initial raw materials are easily available, and the operation is easy.
Owner:SHANGHAI HUMAN BIOTECH CO LTD
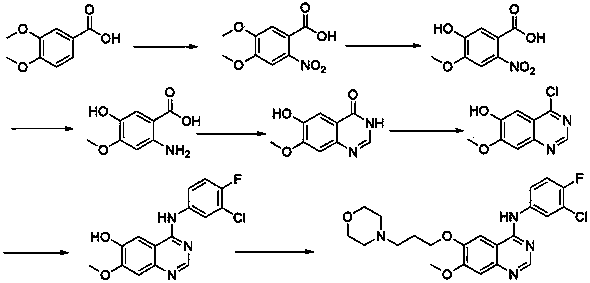
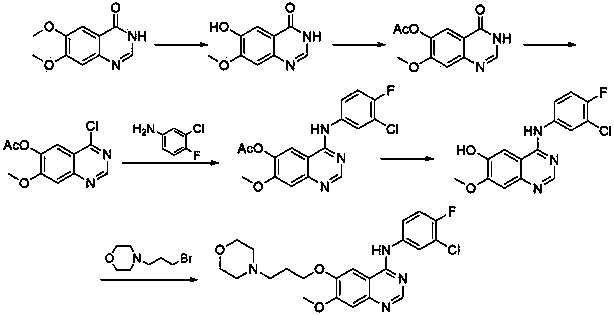
New preparation method of lapatinib
ActiveCN103483324AEfficient manufacturingHigh purityOrganic chemistryN dimethylformamideDimethyl acetal
The present invention provides a preparation method of lapatinib. The method comprises contacting a compound shown as a formula 1 with a compound shown as a formula 2 to produce a compound shown as a formula 3; reducing the compound shown as the formula 3 to produce a compound shown as a formula 4; contacting a compound shown as a formula 5 with N,N-dimethylformamide dimethyl acetal to produce a compound shown as a formula 6; contacting the compound shown as the formula 6 with the compound shown as the formula 4 to produce a compound shown as a formula 7; in the presence of an acid, an alkali and NaNH(OAc)3, contacting a compound shown as a formula 8 with a compound shown as a formula 9 to produce a compound shown as a formula 10; in the presence of a catalyst and an alkali, contacting the compound shown as the formula 10 with a compound shown as a formula 11 to produce a transition intermediate, and contacting the transition intermediate with the compound shown as the formula 7 and p-toluenesulfonic acid to produce a compound shown as a formula I; through use of the method, the lapatinib can be effectively prepared.
Owner:HUBEI BIO PHARMA IND TECHCAL INST
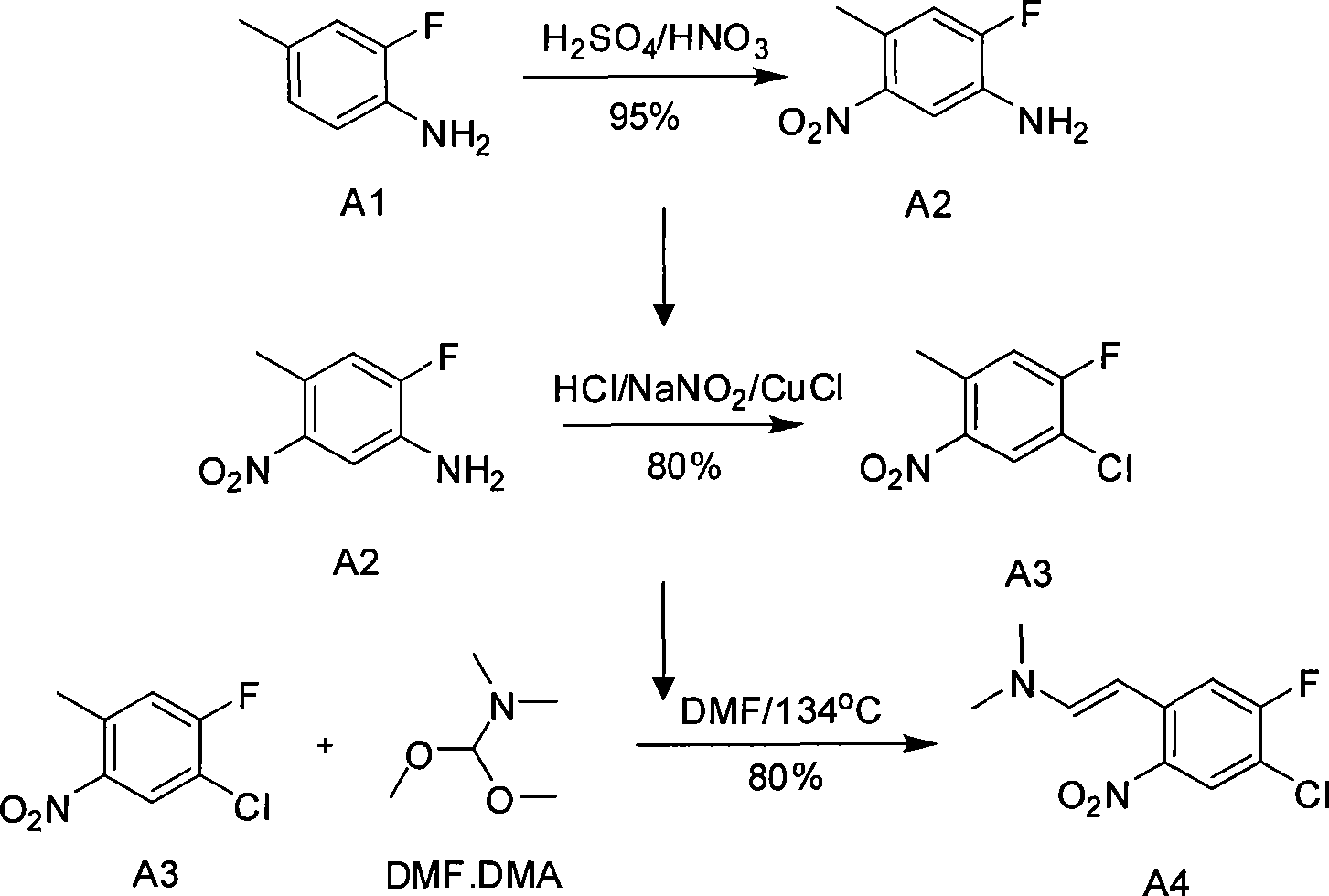
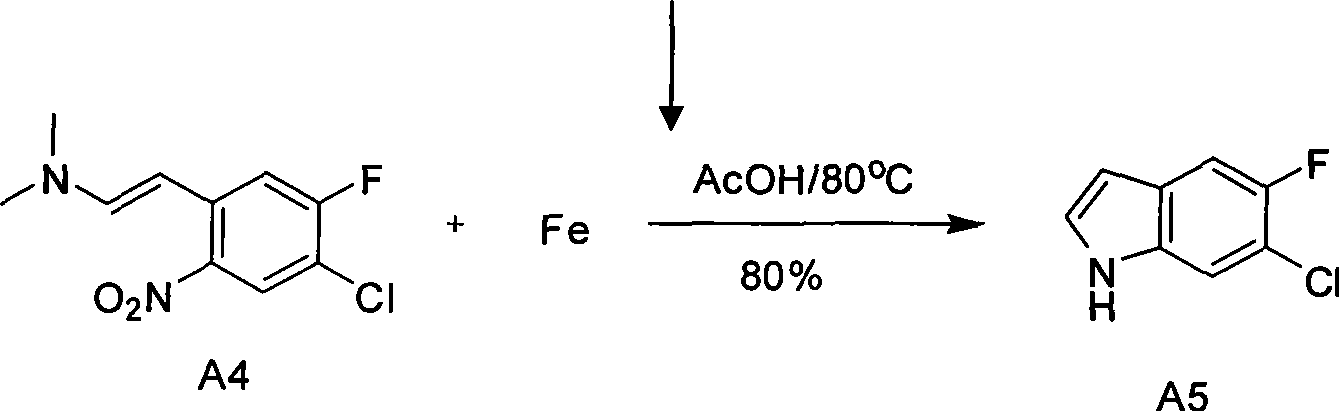
Preparation for medicinal intermediate 6-chloro-5-fluroindole for synthesizing anti-cancer medicament
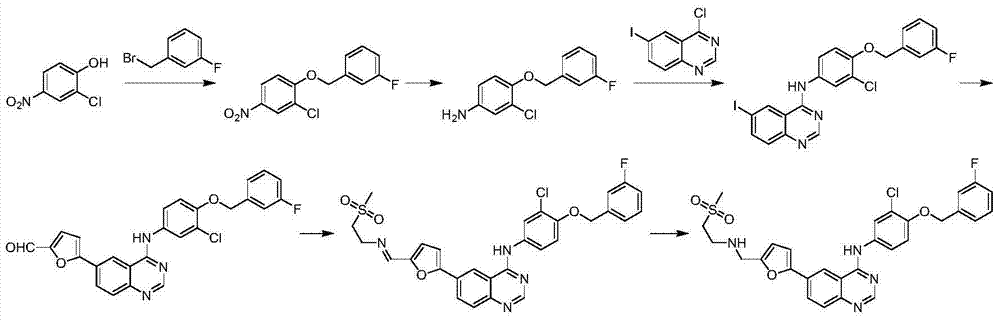
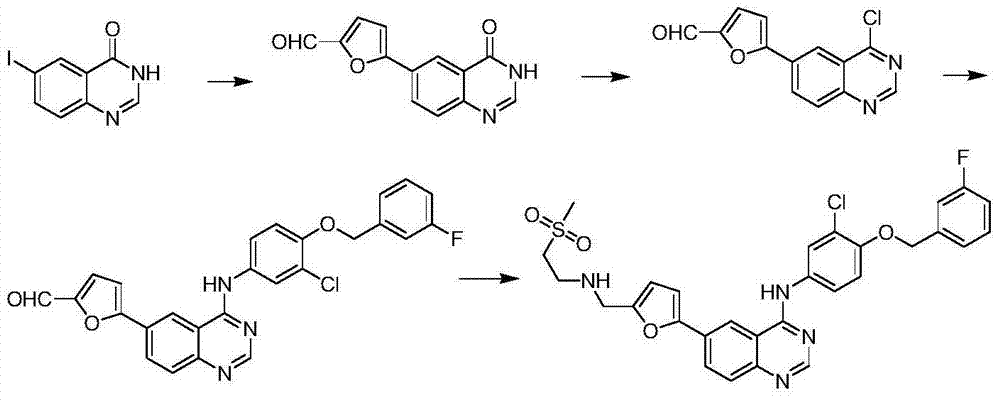
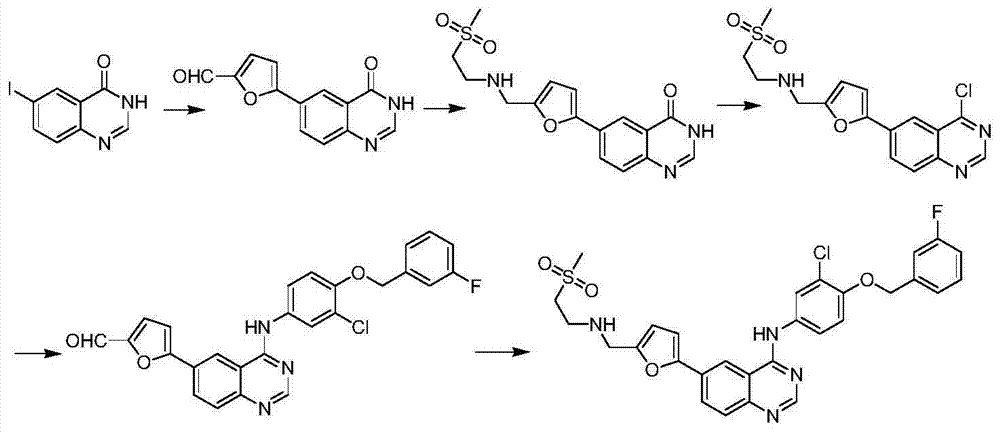
The invention relates to preparation for a medicinal intermediate 6-chloro-5-fluroindole for synthesizing an anti-cancer medicament, which comprises the following steps that: firstly, 3-fluro-4-aminotoluene reacts in a concentrated sulfuric acid system, the pH value is adjusted to be neutral after the reaction, and the mixture is filtered to obtain 3-fluro-4-amino-6-nitrotoluene; secondly, a sodium nitrite solution and cuprous chloride are added in the concentrated sulfuric acid system, and the mixture is diluted by clear water to obtain 3-fluro-4-chloro-6-nitrotoluene; thirdly, the 3-fluro-4-chloro-6-nitrotoluene and N, N-dimethyl formamide dimethylacetal react in a molecular sieve drying DMF system to obtain 2-(methylamino) ethylene-4-fluro-5-chloronitrobenzene; and finally, iron powder is added into an acetic acid system, and after the reaction, the mixture is recrystallized by using a mixed system containing petroleum ether to obtain the white 6-chloro-5-fluroindole. The method has the advantages of simple operation, environmental friendliness, high purity and low cost, and is suitable for industrialized production.
Owner:上海泰坦科技股份有限公司
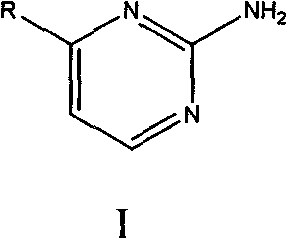
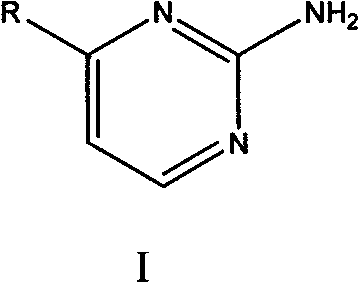
4-substituent-2-amido pyrimidine compound and preparation method thereof
InactiveCN101602757AThe reaction process is simpleMild conditionsOrganic chemistrySodium methoxideAlcohol
The invention relates to a 4-substituent-2-amido pyrimidine compound and a preparation method thereof. The invention adopts a technical scheme of having a structure shown as general formula (I). The preparation method comprises the following steps that: an aromatic ring or heterocyclic ring compound with acetyl radicals is put into a container together with N, N-dimethyl formamide dimethyl acetal, and the mixture is evenly stirred, is heated until refluxing and reacts for 3 to 5 hours, the stirring is stopped, and the mixture is cooled to room temperature, filtered and dried to obtain an intermediate product; sodium ethylate or sodium methoxide is dissolved in absolute alcohol, the mixture is evenly stirred, guanidine hydrochloride is added into the mixture, the obtained mixture is stirred for 0.5 to 1.5 hours at room temperature, and A liquid is obtained; the intermediate product is dissolved in the absolute alcohol to obtain B liquid; and the B liquid is slowly dropped into the A liquid, the reaction system is heated until refluxing, the reaction is carried out for 5 to 7 hours, the stirring is stopped, and the obtained mixture is cooled to room temperature, stands overnight, is filtered, washed and dried. The invention has high yield, high purity, simple technology and universality.
Owner:丹东恒悦新材料有限公司
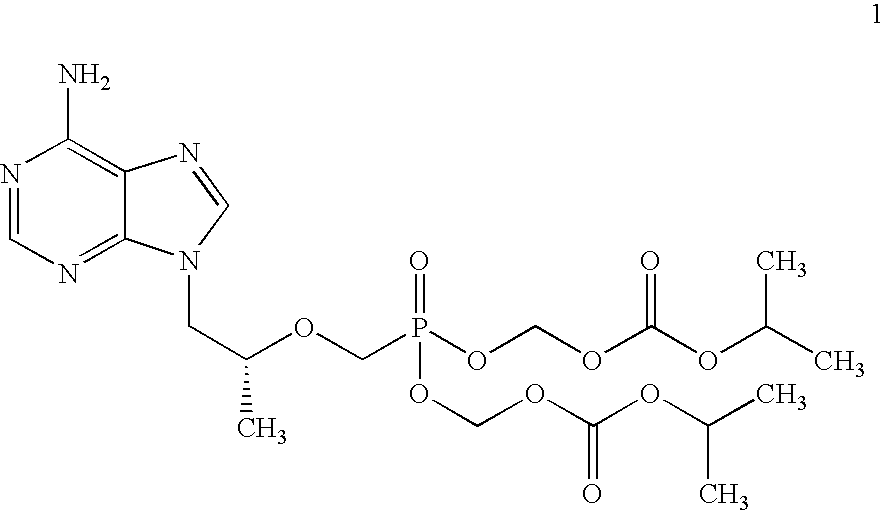
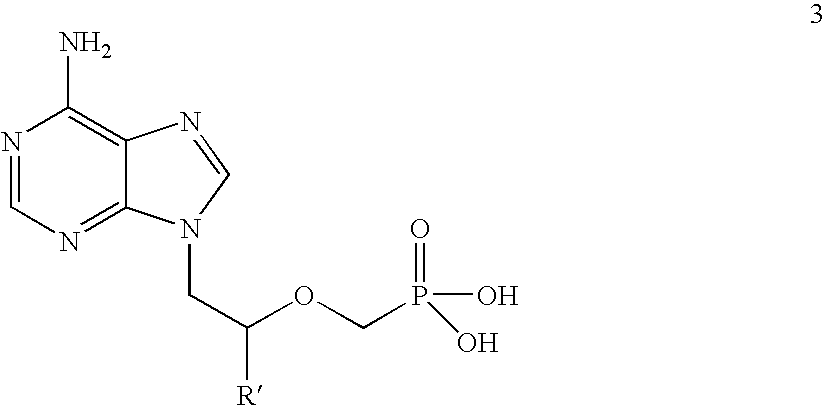
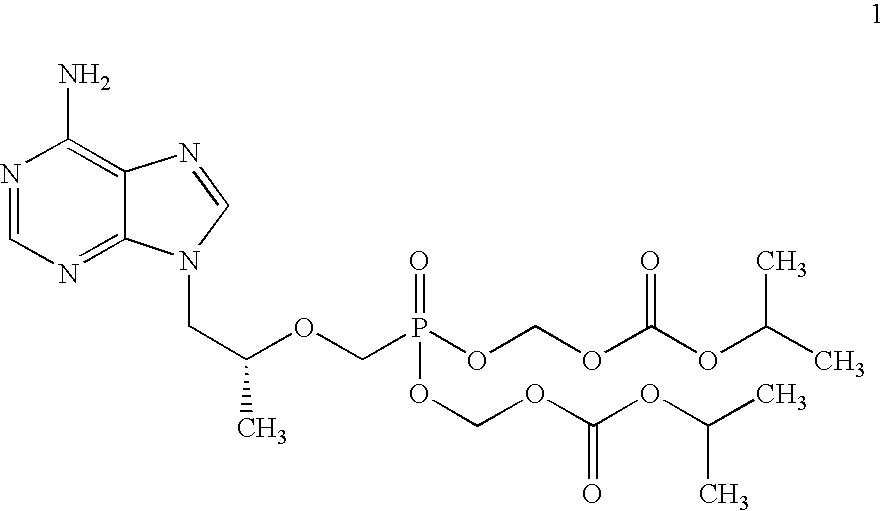
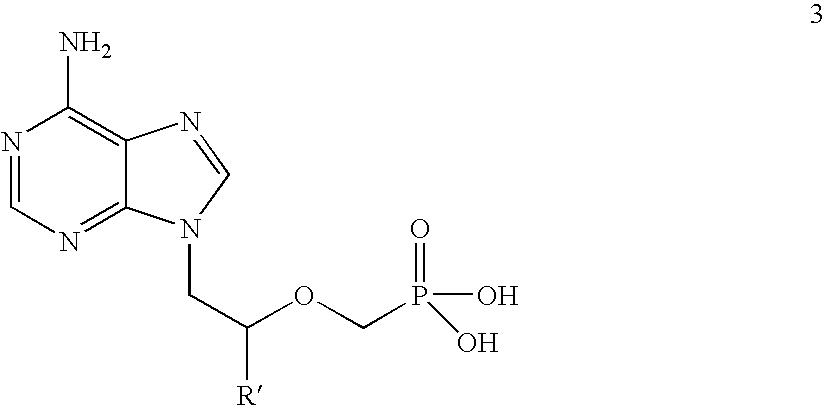
Novel process for acyclic phosphonate nucleotide analogs
The present invention provides a novel process for the preparation of acyclic phosphonate nucleotide analogs using novel intermediates. Thus, for example, (R)-9-(2-phosphonomethoxypropyl)adenine is reacted with dimethylformamide dimethylacetal to give N4-dimethylaminomethyledino-9-(2-phosphonomethoxy ethyl) adenine, which is then reacted with chloromethyl-2-propyl carbonate in presence of triethylamine to give (R)—N4-Dimethylaminomethyledino-9-(2-phosphono methoxypropyl) adenine disoproxil, followed by deprotection with acetic acid to get tenofovir disoproxil. Tenofovir disoproxil is then treated with fumaric acid to give tenofovir disoproxil fumarate.
Owner:HETERO DRUGS LTD
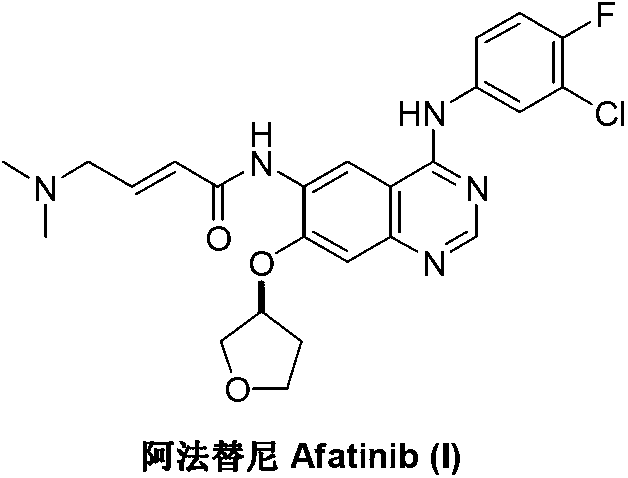
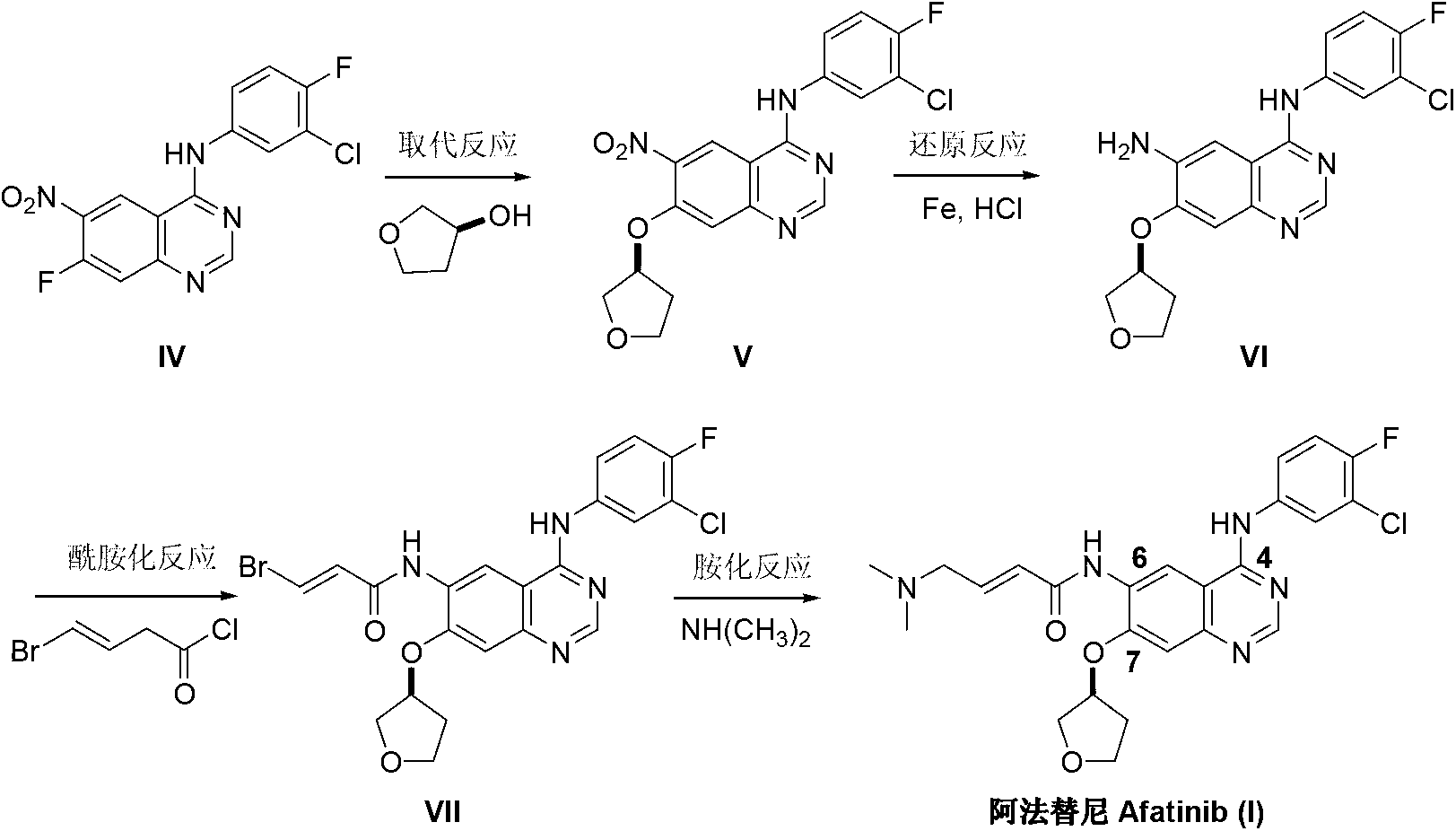
Method for preparing Afatinib
ActiveCN103254183AEase of industrial productionPromote the development of economy and technologyOrganic chemistryN dimethylformamideAniline
The invention discloses a method for preparing Afatinib (I). The method comprises the following steps: 2-cyano-4-[4-(N,N-dimethylamino)-1-oxo-2-buten-1-yl]amino-5-[(S)-(tetrahydrofuran-3-yl)oxy]aniline (II) and N,N-dimethylformamide dimethylacetal (DMF-DMA) are subjected to condensation reaction so as to produce an intermediate (III), and the intermediate (III), without to the need of separation, is directly subjected to cyclization reaction with 4-fluoro-3-chloroaniline so as to prepare Afatinib (I). According to the method, the steps for preparing Afatinib are reduced obviously, and the cost is reduced greatly.
Owner:铜陵尚东高新科创有限公司
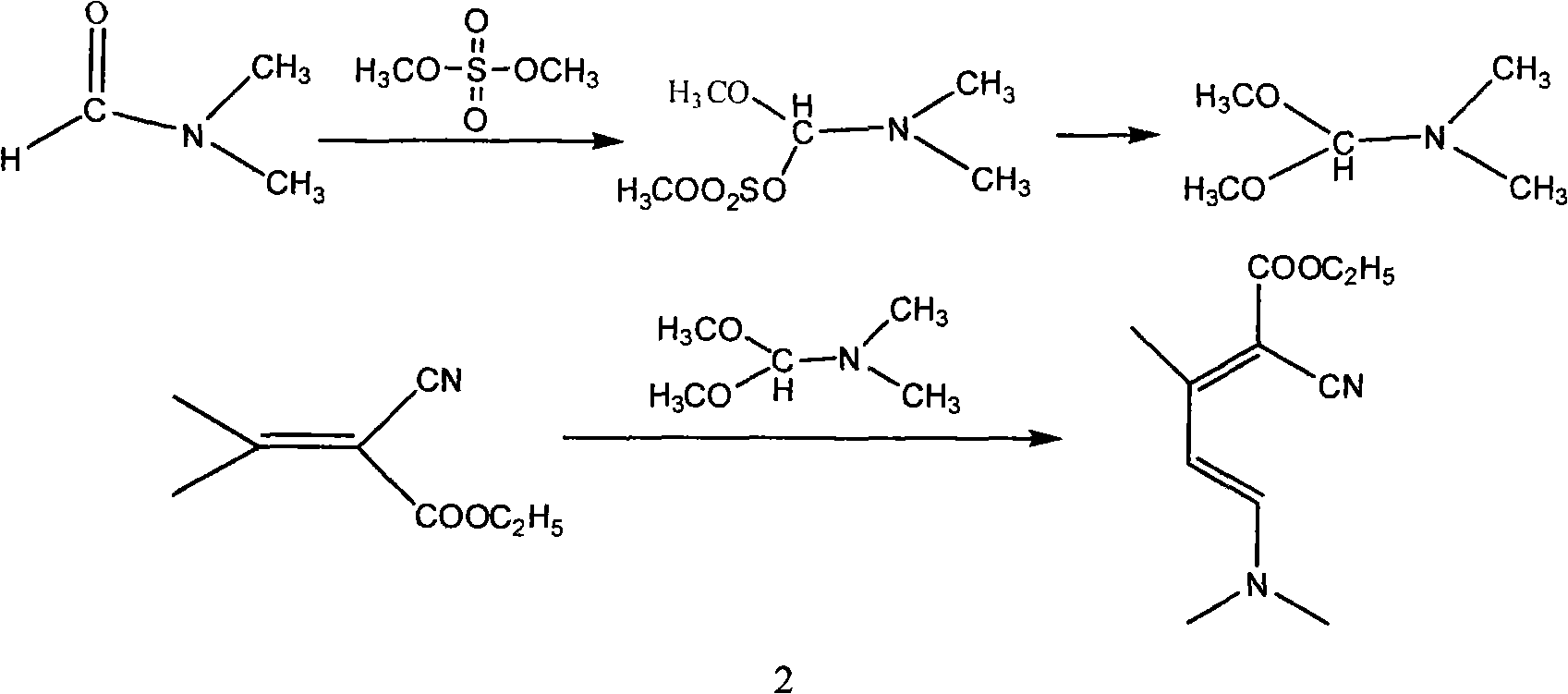
Method for synthesizing 2-chloro-3-amino-4-methylpyridine by ethyl cyanoacetate and acetone
InactiveCN101565399AComplicated operationFew stepsOrganic chemistrySodium methoxideOrganic synthesis
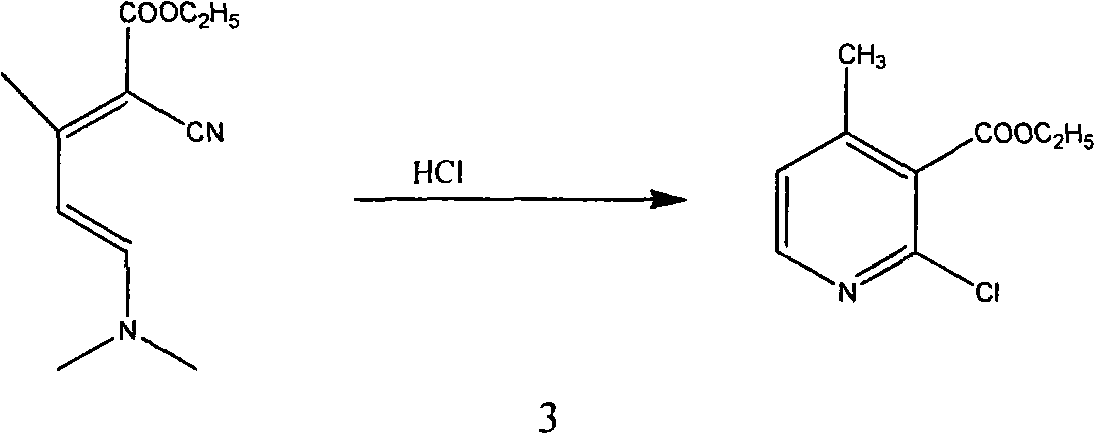
The invention relates to a method for synthesizing an important intermediate 2-chloro-3-amino-4-methylpyridine for an anti-AIDS medicament Nevirapine, and belongs to the technical field of organic synthesis. The method comprises the following process steps that: ethyl cyanoacetate and acetone are dehydrated and condensed to generate a condensation compound I under the action of a catalyst; dimethyl formamide, dimethyl sulfate and sodium methoxide solution react to generate N,N-dimethylformamiade dimethyl acetal (N,N-dimethyl formamide A), and then the N,N-dimethylformamiade dimethyl acetal reacts with the condensation compound I to generate conjugated enamine, namely a condensation compound II; the condensation compound II is cyclized by hydrochloric acid and ethanol to form a cyclic compound 2-chloro-4-methyl-ethyl nicotinate; the 2-chloro-4-methyl-ethyl nicotinate is ammonolyzed by ammonia gas to form 2-chloro-4-methyl-niacinamide; and the 2-chloro-4-methyl-niacinamide is subjected to Hofmann degradation reaction to form the 2-chloro-3-amino-4-methylpyridine. Compared with the prior synthesizing method, the method of the invention has the remarkable characteristic of reducing the reaction steps, and is suitable for large-scale industrialized production; the molar total yield of the five-step reaction is improved to 27 percent from the prior 24 percent; and the purity of the product reaches over 99 percent.
Owner:江苏鼎昊医药科技有限公司
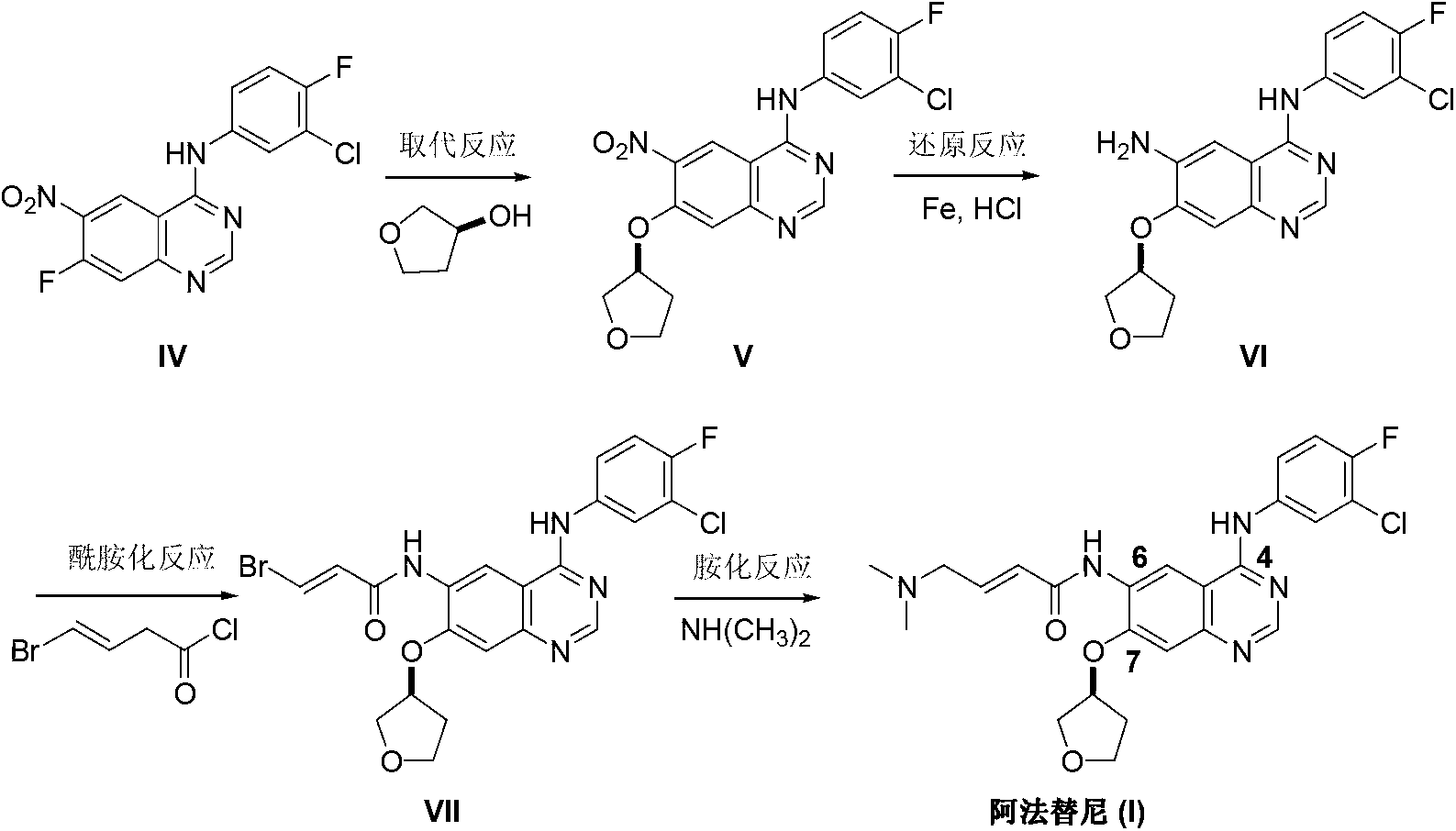
Method for preparing Afatinib
InactiveCN103254182AEase of industrial productionPromote the development of economy and technologyOrganic chemistryN dimethylformamideAniline
The invention discloses a method for preparing Afatinib. The method comprises the following steps: 4-fluoro-3-chloroaniline and N,N-dimethylformamide dimethylacetal (DMF-DMA) are subjected to condensation reaction so as to produce a Schiff base (III), and the Schiff base (III), without to the need of separation, is subjected to cyclization reaction with 2-cyano-4-[4-(N,N-dimethylamino)-1-oxo-2-buten-1-yl]amino-5-[(S)-(tetrahydrofuran-3-yl)oxy]aniline so as to prepare Afatinib. According to the method, the steps for preparing Afatinib are reduced obviously, and the cost is reduced greatly.
Owner:SUZHOU MIRACPHARMA TECH +1
Preparing method of anticancer medicine
The invention discloses a novel synthesis method of an anticancer medicine gefitinib. The preparing method includes subjecting 4-methoxy-5-(3-morpholinopropoxy)-2-nitrobenzonitrile that is adopted as a raw material to reduction by sodium hydrosulfite, salifying with hydrochloric acid, reacting with N,N-dimethylformamide dimethyl acetal to obtain a condensation product, subjecting the condensation product and 3-chloro-4-fluoroaniline to cyclization to obtain the gefitinib. The initial raw material adopted by the preparing method is cheap and easily available. The synthesis route is simplified. The raw material utilization rate and the total yield are largely increased. Reaction intermediates are mostly purified by a recrystallization method or are directly used for a next reaction. The method has characteristics of high yield, less three-waste in reaction processes and low cost, and is suitable for industrial production.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
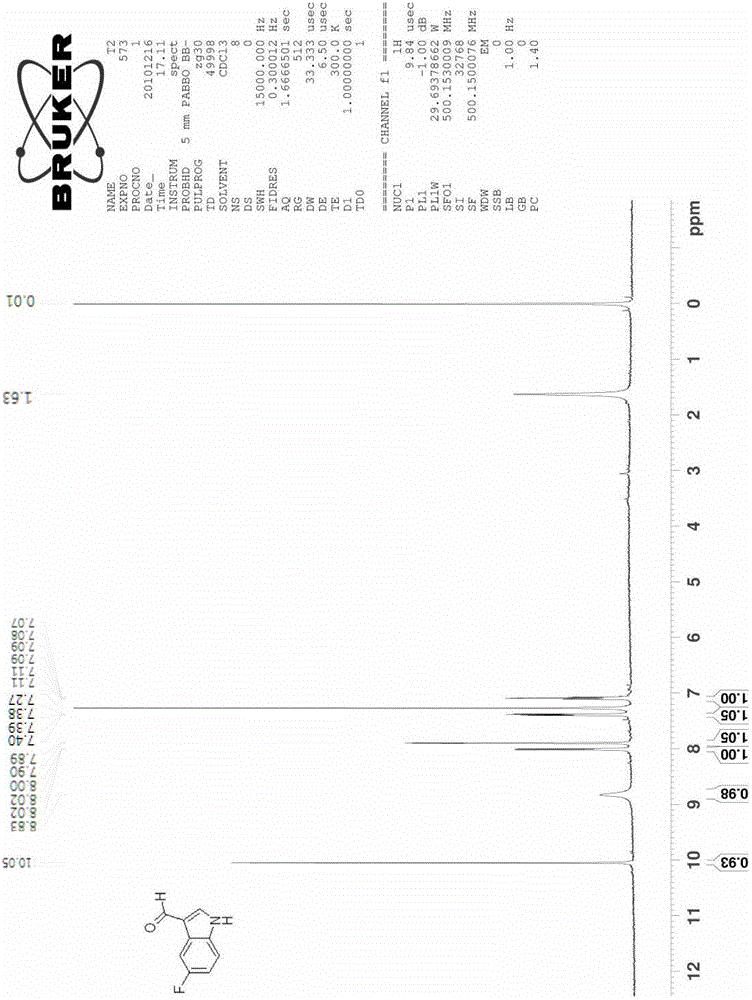
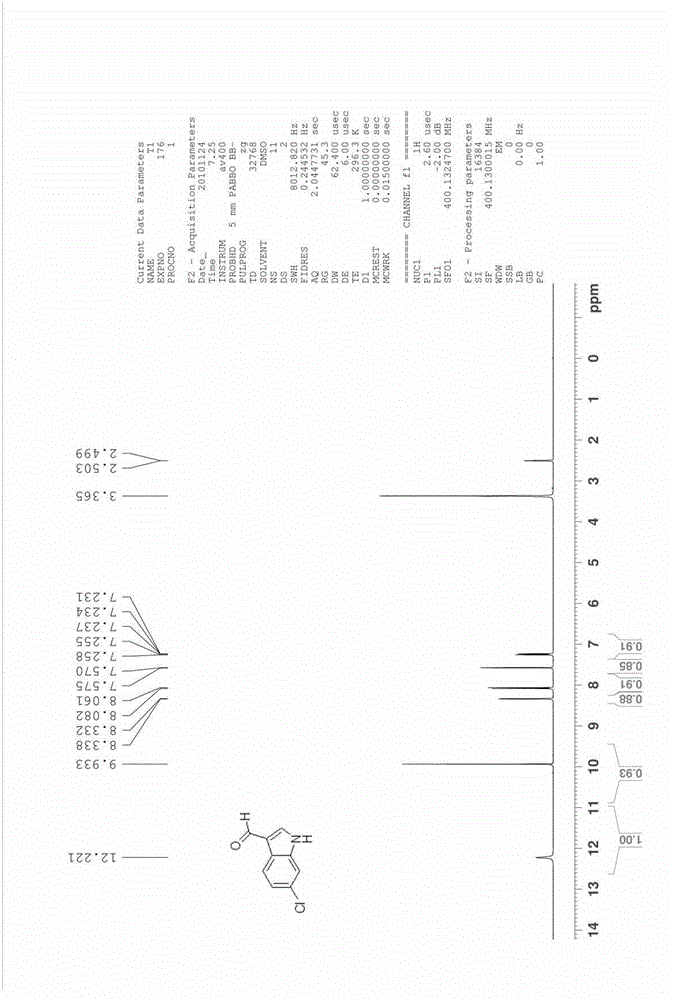
Method for preparing substituted indole-3-methanal compound
The invention relates to the field of organic synthesis and medical intermediate preparation and especially relates to a method for preparing a substituted indole-3-methanal compound. The method comprises the following steps that N,N-dimethylformamidodimethylacetal or N,N-methylformamidodiethylacetal and substituted 2-nitrotoluene undergo a reflux reaction to produce beta-dimethylamino-2-nitrostyrene; beta-dimethylamino-2-nitrostyrene and a mixed solution of hydrazine and alcohol undergo a reaction to produce a substituted indole; and the substituted indole, dimethylformamide (DMF) and phosphorus oxyhalogen undergo a reaction to produce the substituted indole-3-methanal compound. Yields of all processes of the method provided by the invention are more than 90%. A total yield of the method is more than 80%.
Owner:上海泰坦科技股份有限公司
Preparation method of erlotinib hydrochloride crystal form A
ActiveCN103396371AAvoid adjusting pHAvoid the extraction processOrganic chemistryChemical recyclingDimethyl acetalPharmaceutical Substances
The invention provides a preparation method of an erlotinib hydrochloride crystal form A, which belongs to the technical field of the preparation of a drug compound. The preparation method comprises the following steps: enabling 2-amino-4,5-di(2-methoxy ethyoxyl) cyanophenyl and N, N-dimethyl amide dimethyl acetal to react; re-crystallizing and purifying the obtained Schiff base intermediate, and then synthesizing with aminophenylacetylene to obtain erlotinib free alkali; and adding a hydrochloric acid solution, and recrystallizing to obtain the erlotinib hydrochloride crystal form A. According to the scheme of the invention, the process route is short, the product purity is high, the repeatability is good, and the operation is simple and easy to implement, and therefore, the preparation method is suitable for large-scale industrial production.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES
Preparation methods of 1,4,7,10-tetraazacyclododecane and nanofiltration membrane
InactiveCN104387336AImprove interception effectSignificant technological progressMembranesOrganic chemistryN dimethylformamideDodecane
The invention discloses a preparation method of 1,4,7,10-tetraazacyclododecane. The method comprises the following steps: adopting triethylene tetramine, methyl benzene and water to obtain a triethylene tetramine hydrated crystal substance; heating the triethylene tetramine hydrated crystal substance and the methyl benzene to react to obtain a triethylene tetramine-methyl benzene solution, and obtaining linear triethylene tetramine through drying, filtration and reduced pressure distillation; enabling the linear triethylene tetramine, N,N-dimethylformamide dimethylacetal and methyl benzene to react, and crystallizing to obtain clear crystal bis-imidazoline; enabling potassium carbonate, solvent acetonitrile, bis-imidazoline and 1,2-ethylene dibromide to react, and conducting reduced pressure distillation, washing and drying to obtain bromine; under the protection of nitrogen, adding a potassium hydroxide water solution, heating to a back flow state, dropwise adding a bromine salt water solution, and conducting filtration, precipitation, condensation, cooling and crystallization to obtain the 1,4,7,10-tetraazacyclododecane. The invention also provides a preparation method of a polyamide nanofiltration membrane. According to the invention, the purity of 1,4,7,10-tetraazacyclododecane is improved.
Owner:SHANGHAI INST OF TECH
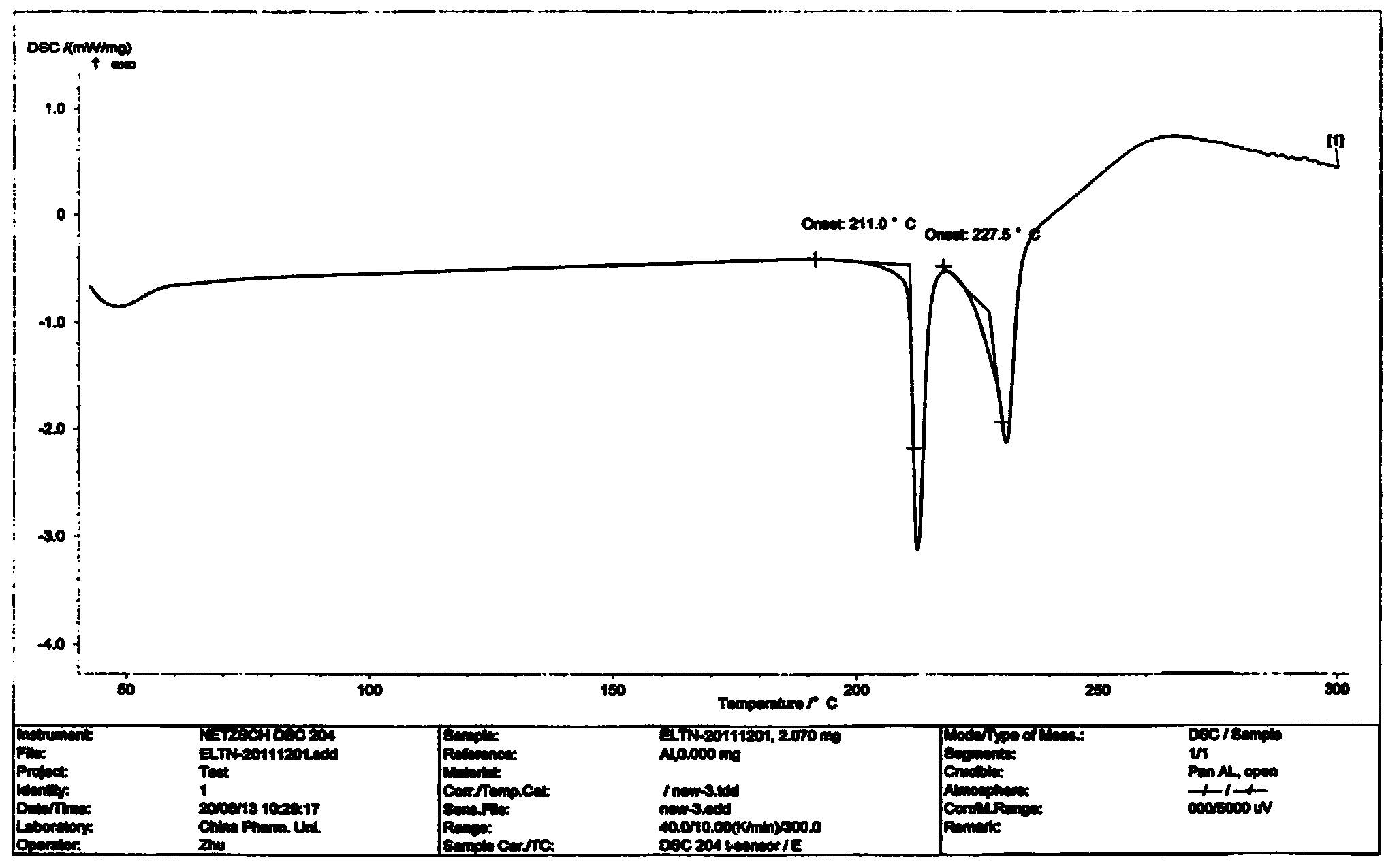
Synthesis method of 6-bromoimidazo[1,2-alpha]pyridyl-3-formic acid
InactiveCN103965191AReaction raw materials are readily availableReasonable priceOrganic chemistry2-amino-5-bromopyridineOrganic synthesis
The invention belongs to the field of organic synthesis, and particularly relates to a synthesis method of 6-bromoimidazo[1,2-alpha]pyridyl-3-formic acid. The method comprises the following steps: reacting N,N-dimethylformamidodimethyl acetal with 2-amino-5-bromopyridine at 40-100 DEG C to obtain an intermediate, and reacting the intermediate with ethyl bromoacetate at 60-160 DEG C for 3-15 hours; after the reaction finishes, cooling to room temperature, and concentrating by rotary evaporation to obtain an ethyl 6-bromoimidazo[1,2-alpha]pyridyl-3-formate crude product; and under the action of an alkali, carrying out hydrolysis reaction on the ethyl 6-bromoimidazo[1,2-alpha]pyridyl-3-formate in a certain solvent for 1-5 hours, neutralizing with hydrochloric acid, filtering, washing with water, and drying to directly obtain the 6-bromoimidazo[1,2-alpha]pyridyl-3-formic acid pure product. The method has the advantages of accessible reaction raw materials, reasonable price, mild reaction conditions and simple after-treatment, and is easy to operate and control; and the product has the advantages of stable quality and high purity.
Owner:SHANDONG YOUBANG BIOCHEM TECH
Synthetic method for lapatinib and lapatinib intermediates
InactiveCN104513231AReduce usageThe synthesis method is simpleOrganic chemistryOrganic solventDimethyl acetal
The invention provides a synthetic method for lapatinib. The method comprises the following steps: (a) in an organic solvent, reacting a compound (6) with a compound (7) in the presence of alkali to obtain a compound (8); (b) in the organic solvent, reacting the compound (8) with a compound (9) in the presence of alkali, and after the reaction is ended, adding a reducing agent for producing a reductive amination reaction to obtain a compound (10); (c) in the organic solvent, reacting the compound (10) with BOC anhydride in the presence of alkali to obtain a compound (11); (d) in the organic solvent, fully reacting the compound (11) with N,N-dimethylformamide dimethyl acetal under the backflow condition, and after the reaction is ended, adding a compound (4) and reacting under the backflow condition to obtain a compound (12); (e) in the organic solvent, producing a deprotection reaction of the compound (12) to obtain lapatinib. The invention further provides two new key intermediates. The method has the characteristics of few steps, high yield and suitability for industrialized production.
Owner:ARROMAX PHARMATECH
Synthesis method of 6-chloroimidazo[1,2-a]pyridine-3-formonitrile
InactiveCN103896941AMild reaction conditionsEasy to operateOrganic chemistryN dimethylformamideSynthesis methods
The invention relates to a synthesis method of 6-chloroimidazo[1,2-a]pyridine-3-formonitrile. The synthesis method of the 6-chloroimidazo[1,2-a]pyridine-3-formonitrile comprises the following steps: reacting 2-amino-5-chloropyridine and N,N-dimethylformamide dimethylacetal at a temperature ranging from 50 to 110 DEG C for 2-10 hours to obtain (E)-N'-(5-chloropyridine-2-yl)-N,N-dimethylformamidine, next, reacting (E)-N'-(5-chloropyridine-2-yl)-N,N-dimethylformamidine with bromoacetonitrile at a temperature ranging from 50 to 150 DEG C for 5-35 hours under the action of a base in a solvent, then carrying out ethyl acetate extraction, water washing, drying and rotary evaporation and concentration to obtain the coarse product of the 6-chloroimidazo[1,2-a]pyridine-3-formonitrile, and recrystallizing the coarse product solvent to obtain the pure product. The synthesis method of the 6-chloroimidazo[1,2-a]pyridine-3-formonitrile has the beneficial effects of mild reaction conditions, easiness for operation, stable product quality and high product purity.
Owner:SHANDONG YOUBANG BIOCHEM TECH
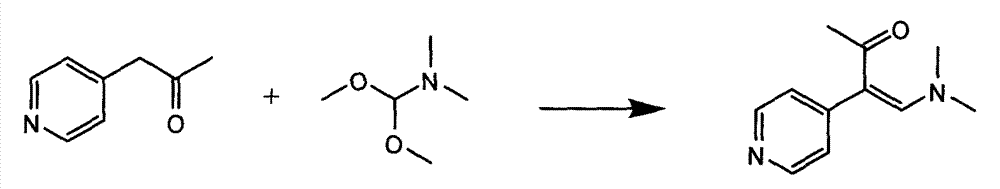
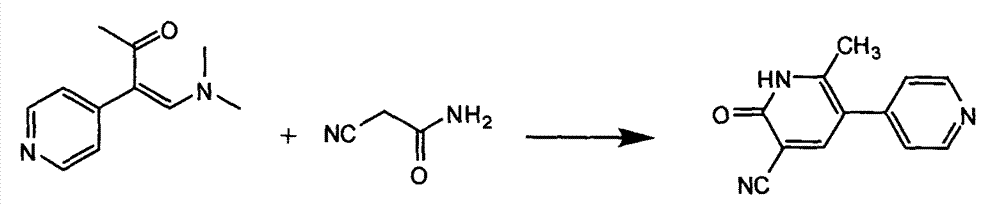
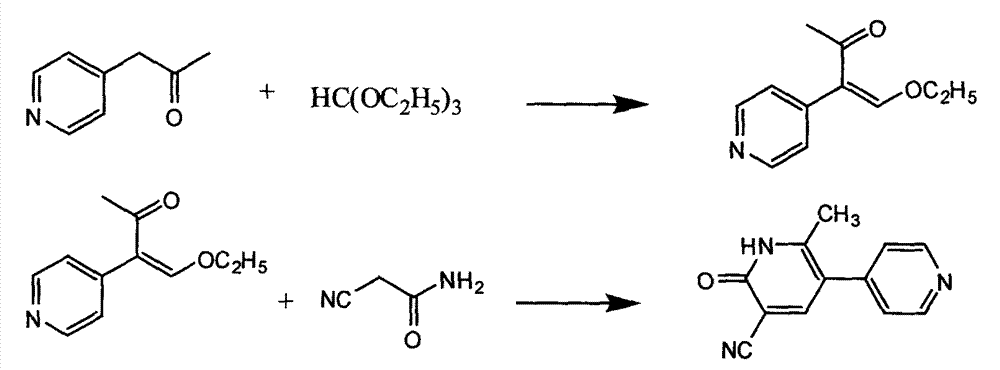
Preparation method of high-purity milrinone
InactiveCN104326975AMild preparation conditionsSimple and fast operationOrganic chemistryDimethyl acetalMethanol
The invention relates to a preparation method of high-purity milrinone as shown in a formula (I) described in the specification. The preparation method comprises the following steps: carrying out reflux reaction on N, N-methyl formamide dimethyl acetal (DMF-DMA) by taking 1-(4-pyridyl) acetone as a starting material, concentrating after the reaction is ended, adding normal hexane into residues to separate out solids to obtain an intermediate 1-(4-pyridyl)-2-(dimethylamino) vinyl methyl ketone; carrying out reflux reaction on the intermediate 1-(4-pyridyl)-2-(dimethylamino) vinyl methyl ketone and cyanoacetamide in the presence of sodium methylate, and regulating the pH value after the reaction is ended to obtain crude milrinone; and refining the crude milrinone by methanol / sodium methylate and alcohol / water to obtain a qualified milrinone product. The preparation method disclosed by the invention is simple in process operation, relatively small in pollution and suitable for industrial production.
Owner:ZHENGZHOU SIHUAN MEDICINE ARTICLE CO LTD
Preparation method of N,N-dimethyl-3-hydroxy-3-aryl propyl amine
InactiveCN1785959AEasy to makeEasy to operateOrganic compound preparationAmino compound preparationN dimethylformamideDimethyl acetal
The present invention discloses a preparation method of N,N-dimethyl-3-hydroxy-3-arylpropylamine. Said method includes the following steps: adding phenyl methyl ketone or 2-acetyl thiophenol and N,N-dimethylformamide dimethyl acetal into solvent including benzene and N,N-dimethylformamide, after the reaction is completed, evaporating solvent, cooling to obtain yellow crystal product, using solvent of ethyl acetate, etc, to make recrystallization and obtain N,N-dimethyl-3-oxo-3-aryl propyleneamine; adopting lithium aluminium hydride as reducing agent, adding N,N-dimethyl-3-oxo-3-aryl propyleneamine and reducing agent into the solvents of benzene, etc., making reaction to obtain product; or adopting sodium borohydride as reducing agent, adding N,N-dimethyl-3-oxo-3-aryl propyleneamine and reducing agent into solvents of acetic acid, etc., making reaction to obtain product; making said product undergo the process of phase separation, evaporating the organic phase obtained by means of phase separation so as to obtain N,N-dimethyl-3-hydrox-3-arylpropylamine.
Owner:TIANJIN UNIV
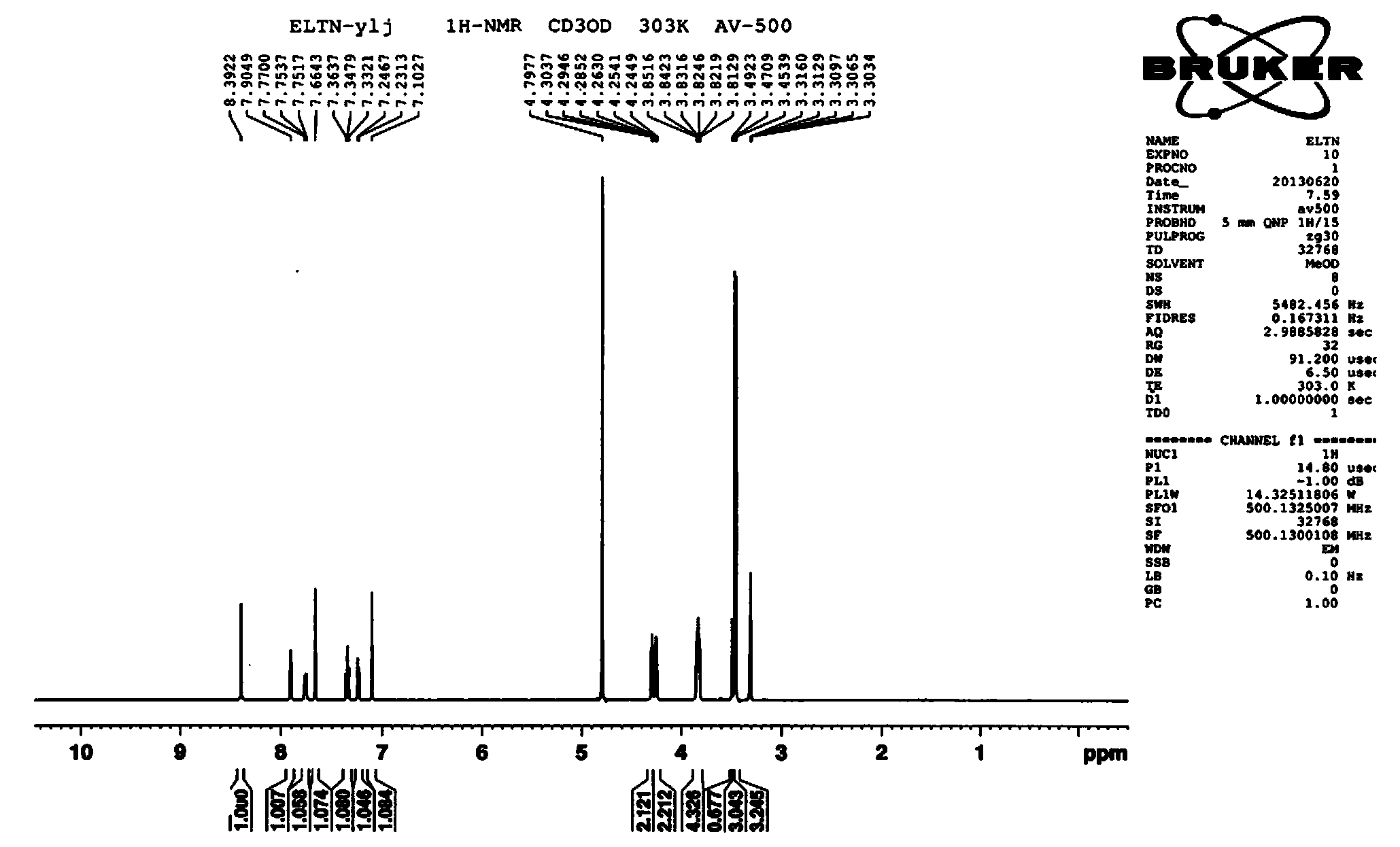
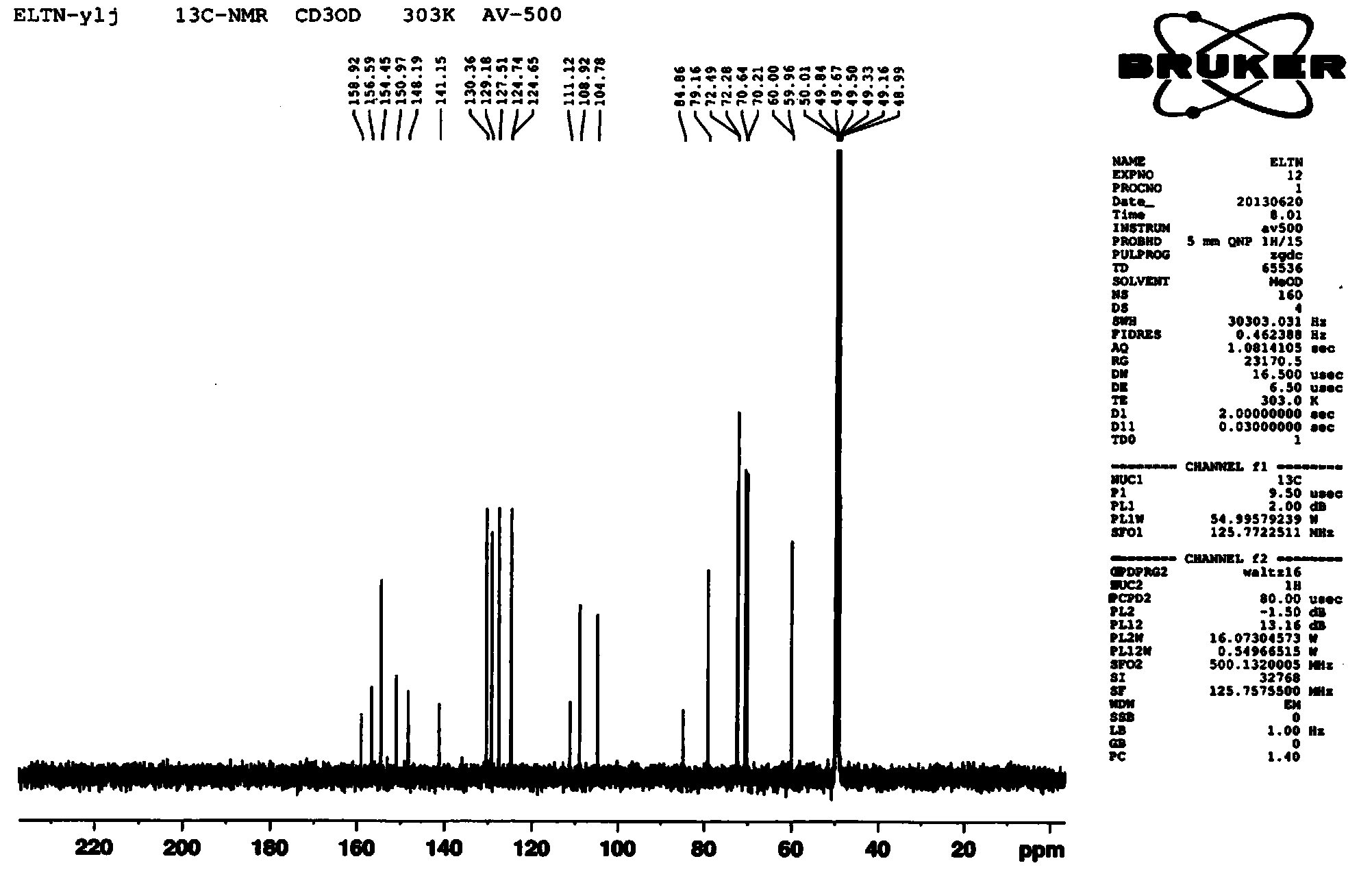
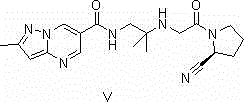
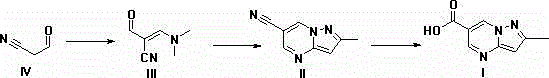
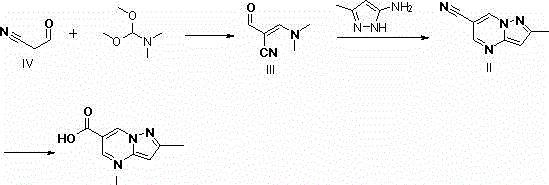
Synthesis method of Anagliptin key intermediate
The invention discloses a synthesis method of Anagliptin key intermediate. The synthesis method comprises the following steps: jointing cyanoacetaldehyde with N,N-dimethylformamide dimethyl acetal to obtain (2E)-3-(dimethylamino)-2-formylacrylonitrile, further performing ring closing with 3-amino-5-methylpyrazole to obtain 2-methyl-pyrazolo[1,5-a]pyrimidine-6-carbonitrile, and then hydrolyzing to obtain the Anagliptin key intermediate. The invention relates to a brand-new method for synthesizing 2-methyl-pyrazolo[1,5-a]pyrimidine-6-carboxylic acid, and the method has the advantages of use of raw materials, less side products, high product purity, and low whole cost.
Owner:安徽安腾药业有限责任公司
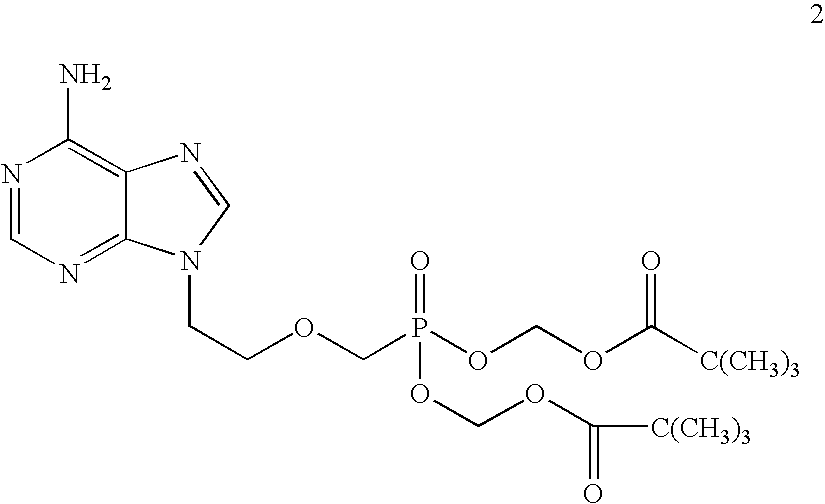
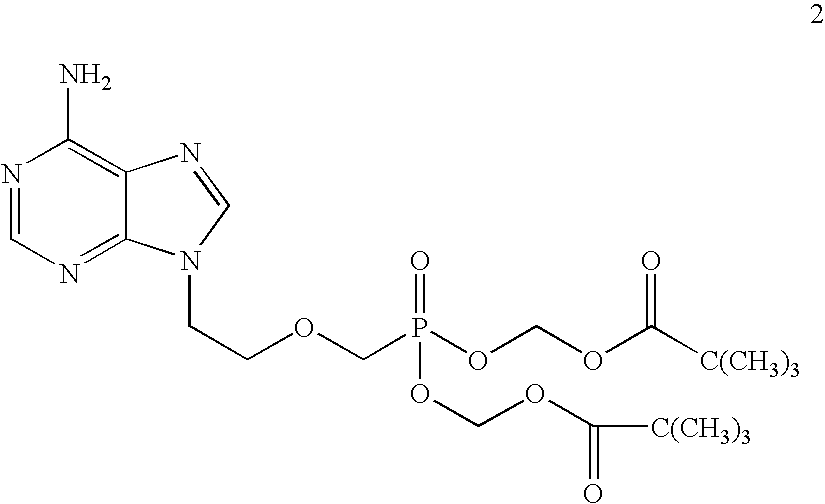
Process for acyclic phosphonate nucleotide analogs
The present invention provides a novel process for the preparation of acyclic phosphonate nucleotide analogs using novel intermediates. Thus, for example, (R)-9-(2-phosphonomethoxypropyl)adenine is reacted with dimethylformamide dimethylacetal to give N4-dimethylaminomethyledino-9-(2-phosphonomethoxy ethyl) adenine, which is then reacted with chloromethyl-2-propyl carbonate in presence of triethylamine to give (R)-N4-Dimethylaminomethyledino-9-(2-phosphono methoxypropyl) adenine disoproxil, followed by deprotection with acetic acid to get tenofovir disoproxil. Tenofovir disoproxil is then treated with fumaric acid to give tenofovir disoproxil fumarate.
Owner:HETERO DRUGS LTD
Synthetic method of ethyl 3-aldehyde-6-chloroimidazo[1,2-a]pyridine-8-formate
InactiveCN104402882AReaction raw materials are readily availableReasonable priceOrganic chemistryN dimethylformamideN-Chlorosuccinimide
The invention relates to a synthetic method of ethyl 3-aldehyde-6-chloroimidazo[1,2-a]pyridine-8-formate. The method comprises the following steps: carrying out a substitution reaction on ethyl 2-aminonicotinate and N-chlorosuccinimide in a certain solvent at normal temperature to prepare ethyl 2-amino-5-chloronicotinate; and reacting2-amino-5-chloronicotinate with N,N-dimethylformamide dimethyl acetal to prepare an intermediate, reacting the intermediate with chloroacetaldehyde in a certain solvent at 0-100DEG C without purifying the intermediate, cooling, and drying to obtain ethyl 3-aldehyde-6-chloroimidazo[1,2-a]pyridine-8-formate. The method has the advantages of easily available reaction raw materials, reasonable price, mild reaction conditions, easy operation, easy control and simple post-treatment, and the above obtained product has the advantages of stable quality and high purity.
Owner:SHANDONG YOUBANG BIOCHEM TECH
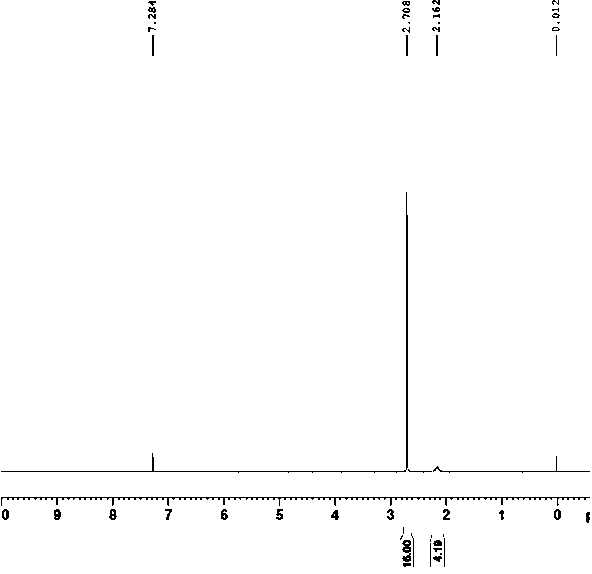
Pyrazolo[1,5-a]pyridine-3-carboxylate derivative synthetic method
InactiveCN107163043AMild reaction conditionsHigh selectivityOrganic chemistryN dimethylformamideOrganic synthesis
The invention relates to the field of organic synthesis, and discloses a method for synthesizing a pyrazolo[1,5-a]pyridine-3-carboxylate derivative. The method comprises the following step: 1) 2-pyridine acetate or substitute 2-pyridine acetate and N,N-dimethylformamide dimethyl acetal are subjected to a reaction; 2) hydroxylamine hydrochloride is continuously added for a reaction to obtain a compound III; and 3) the compound III and trifluoroactic anhydride are subjected to the reaction to generate the product. After the reaction is completed, water is added, and the pH value is adjusted to neutrality, extraction is carried out and the organic phases are merged, and then the steps of drying, concentration and re-crystallization are carried out. The synthetic method has the advantages of easy acquisition of the raw materials, mild reaction condition and high yield; whether the raw materials have a symmetrical structure or not, the target product can be specifically obtained, post-treatment and purifying are easily operated, and amplification production can be realized.
Owner:上海毕得医药科技股份有限公司
Preparation method of 5-(tert-butyloxycarbonyl)-2-methyl-4,5,6,7-tetrahydro-2H-pyrazolo[4,3-C]pyridine-7-carboxylic acid
InactiveCN110551123AMethod route shortHigh yieldOrganic chemistryN dimethylformamideTert-Butyloxycarbonyl protecting group
The invention relates to a preparation method of 5-(tert-butyloxycarbonyl)-2-methyl-4,5,6,7-tetrahydro-2H-pyrazolo[4,3-C]pyridine-7-carboxylic acid, and mainly solves the technical problem that a method suitable for industrial synthesis does not exist at present. The method comprises four steps: firstly, carrying out a reaction of a compound 1 and N,N-dimethylformamide dimethylacetal to obtain a compound 2; then, carrying out a reaction of the compound 2 and hydrazine hydrate in ethanol to obtain a compound 3; carrying out a reaction of the compound 3 with methyl iodide in an ethyl acetate solvent and with cesium carbonate as an alkali, to generate a compound 4; and finally, hydrolyzing the compound 4 with sodium hydroxide in water and ethanol to obtain a target compound 5. The obtained compound is a useful intermediate or product for synthesizing many medicines.
Owner:CHANGZHOU HEQUAN PHARMA CO LTD
Synthesis method of imidazo[1,2-alpha]pyridyl-3-formic acid
InactiveCN103965190AReaction raw materials are readily availableReasonable priceOrganic chemistrySynthesis methodsOrganic synthesis
Owner:SHANDONG YOUBANG BIOCHEM TECH
Preparation technology for 3-aryl-4-nitro isoxazole compound
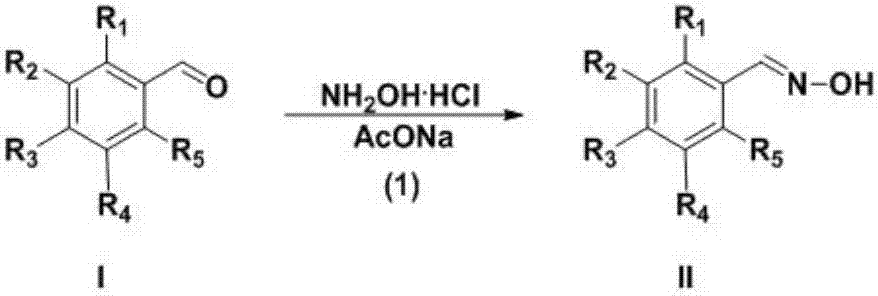
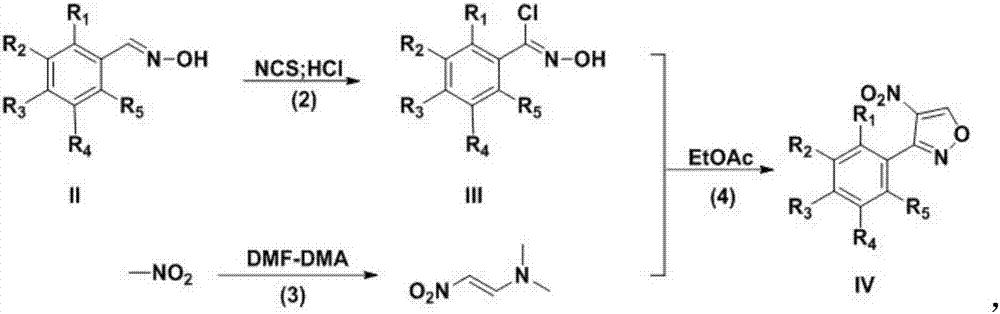
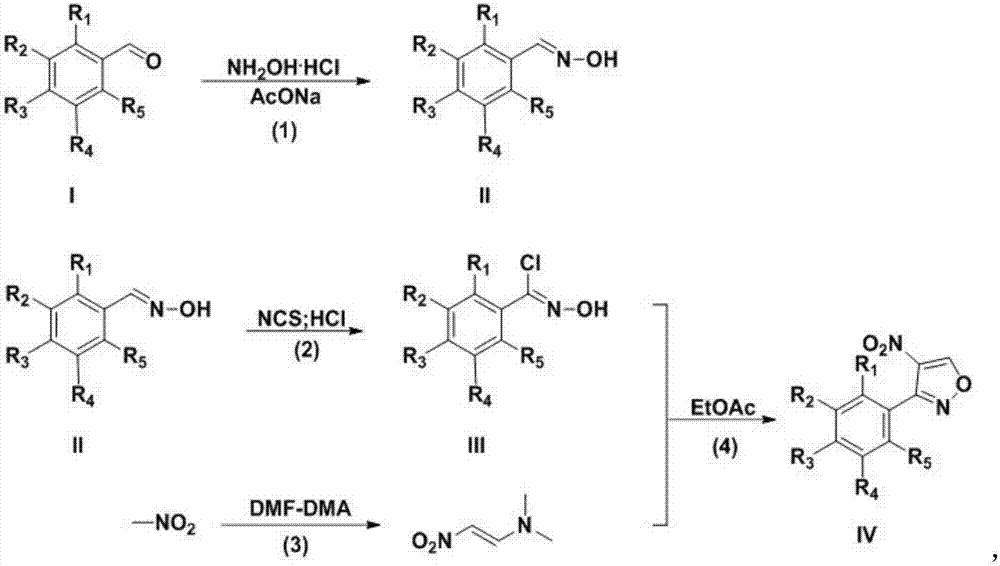
InactiveCN107382892ARaw materials are cheap and easy to getEasy to purifyOrganic chemistryN dimethylformamideDimethyl acetal
The invention discloses a preparation technology for a 3-aryl-4-nitro isoxazole compound. The preparation technology comprises the following steps: synthesizing a compound shown as formula II through the nucleophilic addition of hydroxylamine hydrochloride and the compound shown as formula I used as the raw material; acquiring the compound shown as formula III through the substitution reaction of the compound shown as formula II and N-chlorosuccinimide; preparing 1-dimethyl amino-2-nitro ethylene through the reaction of N,N-dimethylformamide dimethyl acetal and nitromethane used as the raw material; and acquiring a target product 3-aryl-4-nitro isoxazole compound through the cyclization reaction of the compound shown as formula III and 1-dimethyl amino-2-nitro ethylene. The raw materials in the synthesis route are low in cost and easily acquired, the operation condition is mild and is easily controlled, the product is easily purified and the preparation technology is a new method for synthesizing the 3-aryl-4-nitro isoxazole compound.
Owner:GUIZHOU UNIV
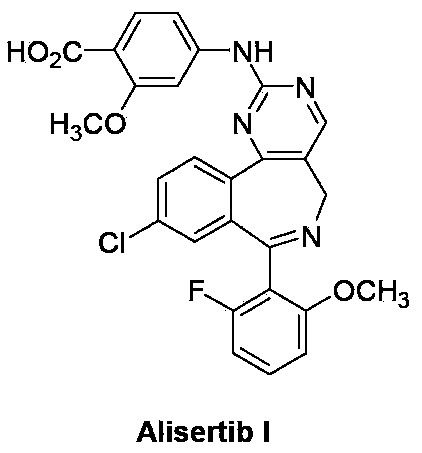
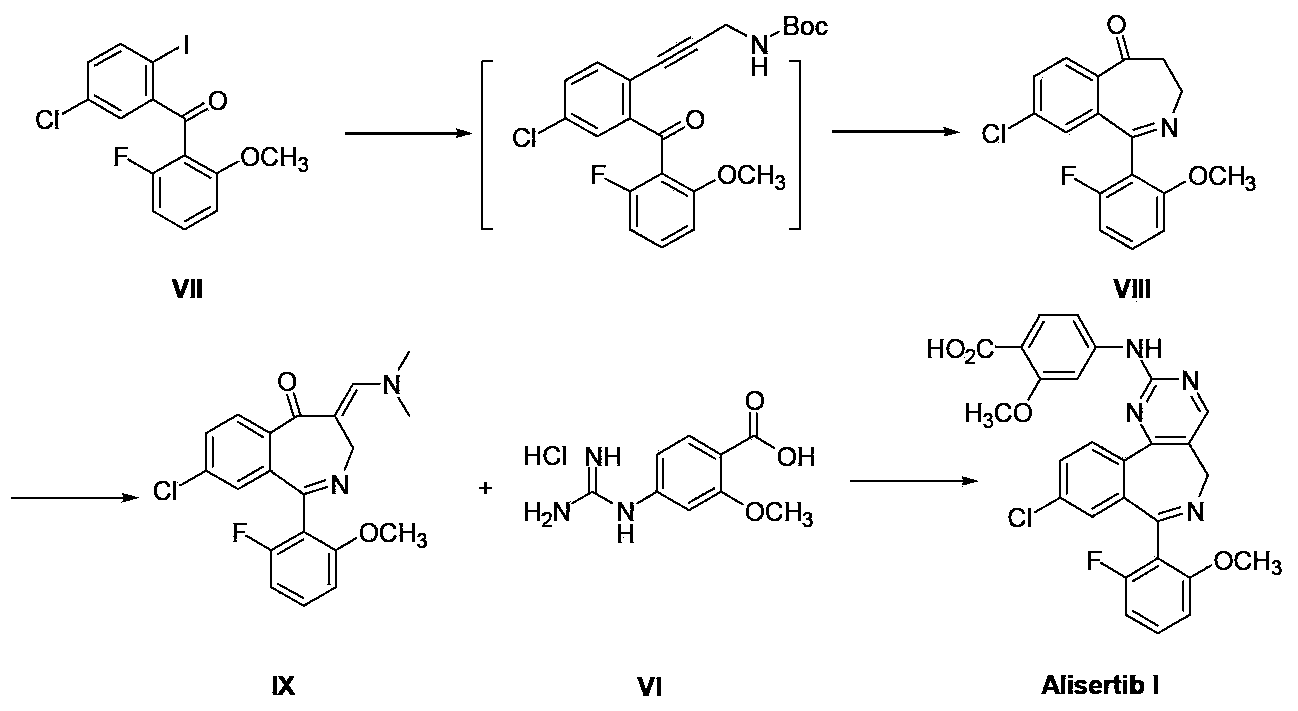
Method for preparing Alisertib
ActiveCN103408552ARaw materials are easy to getSimple processOrganic chemistryIsobenzofuranPropionitrile
The invention discloses a method for preparing Alisertib (I), which comprises the following steps: an addition reaction is carried out between 3-[(2-fluoro-6-methoxyl) phenyl]-5-chlorobenzene phthalein (II) and acetonitrile, so as to generate 1,3-dihydro-1-hydroxyl-3-[(2-fluoro-6-methoxyl) phenyl]-5-chloro-isobenzofuranone-1-acetonitrile (III); a condensation reaction is carried out between the intermediate (III) and N,N-dimethyl formamide-dimethylacetal (DMF-DMA), so as to generate 3-{[1-(4-chlorphenyl)-1'-(2-fluoro-6-methoxyphenyl) methanol]-2-group}-2-[2-(dimethylamino) methene]-3-oxa-propionitrile (IV); a cyclization reaction is carried out between the intermediate (IV) and 4-guanidine-2-methoxyl benzoate hydrochloride (VI), so as to generate 4-{[4-[1-(4-chlorphenyl)-1'-(2-fluoro-6-methoxyphenyl) methanol]-5-pyrimidine-carbonitrile-2-group] amino}-2-methoxyl benzoic acid (V); Alisertib (I) is obtained by reducing, oxidizing and cyclizing the intermediate (V). The method has the advantages that the process is easy; the raw materials are easy to obtain; the requirement for industrial magnification is met.
Owner:临泉县联正电子商务有限公司
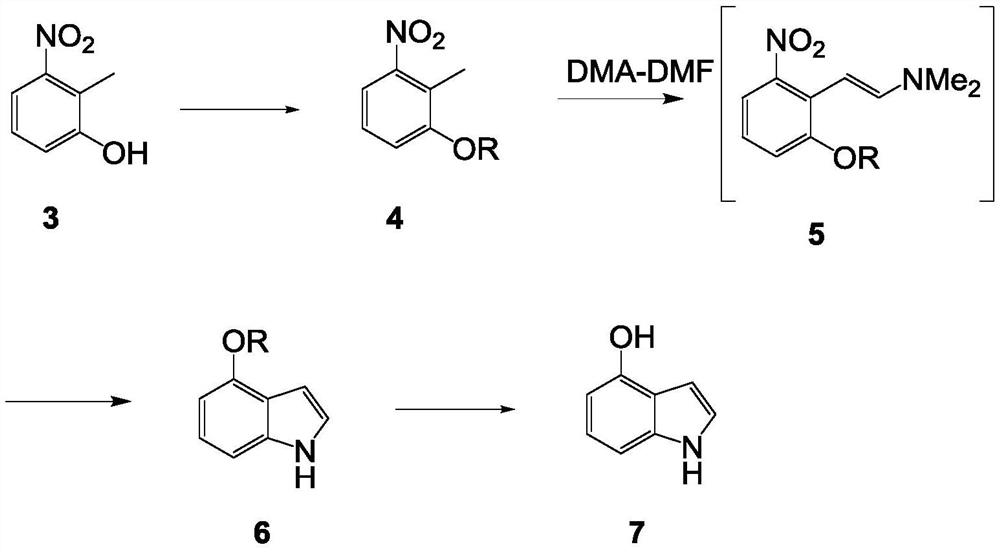
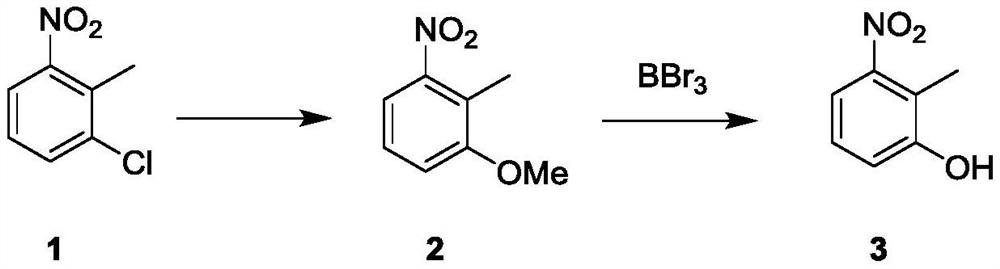
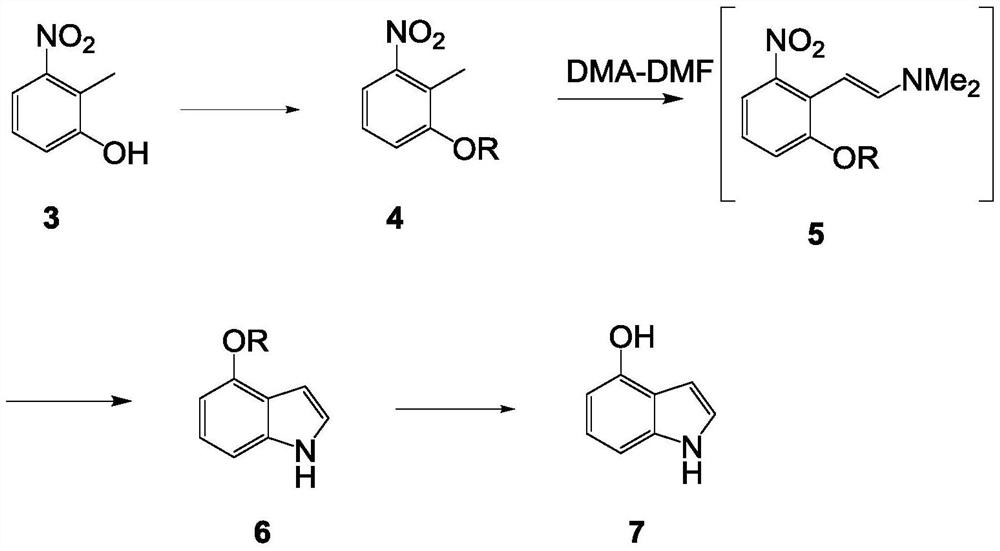
Synthetic method of 4-hydroxyindole
InactiveCN112552224ALow priceMild reaction conditionsOrganic chemistryBulk chemical productionOrganic synthesisDimethyl acetal
The invention belongs to the technical field of organic synthesis, and particularly relates to a synthetic method of 4-hydroxyindole. Aiming at the problems existing in industrial production of 4-hydroxyindole in the prior art, the technical scheme of the invention is as follows: the method comprises the following steps: (1) protecting hydroxyl in a compound 3 by using a protective group to obtaina compound 4; (2) reacting the compound 4 with N, N-dimethylformamide dimethyl acetal to obtain a compound 5; (3) mixing the compound 5 with NH2NH2.H2O and MeOH, and carrying out a reaction to obtaina compound 6; and (4) removing the protective group of the compound 6 to obtain 4-hydroxyindole. According to the method for synthesizing 4-hydroxyindole, the cost of starting materials is low, the reaction conditions in the synthesis process are mild, operation is convenient, aftertreatment is simple, and the yield is high.
Owner:ASTATECH CHENGDU BIOPHARM CORP
Synthetic method for imidazo-[1, 2a]-3,8-PET
InactiveCN104876926AReaction raw materials are readily availableReasonable priceOrganic chemistryN dimethylformamideOrganic synthesis
The invention relates to the field of organic synthesis, and particularly relates to a synthetic method for imidazo-[1, 2a]-3,8-PET, which comprises the following steps of reacting N, N-dimethylformamide dimethyl acetal with ethyl 2-aminopyridine-3-carboxylate at 40-100 DEG C to obtain a purification-free intermediate. The intermediate and ethyl bromoacetate, in a certain proportion, react with each other at 50-160 DEG C in a certain solvent under the effect of alkali. When the reaction is finished, the obtained product is cooled to the room temperature, extracted in the ethyl acetate solution, washed in water and NaCl saturated solution, dried by anhydrous sodium sulfate, rotated, evaporated and concentrated to obtain an imidazo-[1, 2a]-3,8-PET primary product. The imidazo-[1, 2a]-3,8-PET primary product is recrystallized in a mixed solution of n-hexane and ethyl acetate in the volume ratio of 1:1, and then a pure product can be obtained. The above method is easily available in reaction raw material, reasonable in price, mild in reaction condition, easy to operate, easy to control and simple in post-treatment. Products obtained through the above method are stable in quality and high in purity.
Owner:SHANDONG YOUBANG BIOCHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
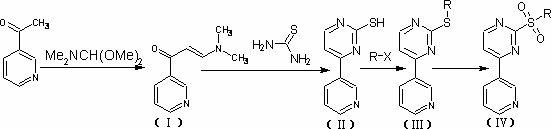

![Synthesis method of 6-bromoimidazo[1,2-alpha]pyridyl-3-formic acid Synthesis method of 6-bromoimidazo[1,2-alpha]pyridyl-3-formic acid](https://images-eureka.patsnap.com/patent_img/e1ecd4ae-f3a6-4447-886a-cb494a9c350b/201410212550X100002DEST_PATH_IMAGE001.PNG)
![Pyrazolo[1,5-a]pyridine-3-carboxylate derivative synthetic method Pyrazolo[1,5-a]pyridine-3-carboxylate derivative synthetic method](https://images-eureka.patsnap.com/patent_img/0eca9911-94f4-4928-ac21-ce309a54fea8/BDA0001324488790000021.png)
![Pyrazolo[1,5-a]pyridine-3-carboxylate derivative synthetic method Pyrazolo[1,5-a]pyridine-3-carboxylate derivative synthetic method](https://images-eureka.patsnap.com/patent_img/0eca9911-94f4-4928-ac21-ce309a54fea8/BDA0001324488790000031.png)
![Pyrazolo[1,5-a]pyridine-3-carboxylate derivative synthetic method Pyrazolo[1,5-a]pyridine-3-carboxylate derivative synthetic method](https://images-eureka.patsnap.com/patent_img/0eca9911-94f4-4928-ac21-ce309a54fea8/BDA0001324488790000051.png)
![Preparation method of 5-(tert-butyloxycarbonyl)-2-methyl-4,5,6,7-tetrahydro-2H-pyrazolo[4,3-C]pyridine-7-carboxylic acid Preparation method of 5-(tert-butyloxycarbonyl)-2-methyl-4,5,6,7-tetrahydro-2H-pyrazolo[4,3-C]pyridine-7-carboxylic acid](https://images-eureka.patsnap.com/patent_img/6279f714-9832-411c-af44-17d61e75466c/DEST_PATH_RE-S.png)
![Preparation method of 5-(tert-butyloxycarbonyl)-2-methyl-4,5,6,7-tetrahydro-2H-pyrazolo[4,3-C]pyridine-7-carboxylic acid Preparation method of 5-(tert-butyloxycarbonyl)-2-methyl-4,5,6,7-tetrahydro-2H-pyrazolo[4,3-C]pyridine-7-carboxylic acid](https://images-eureka.patsnap.com/patent_img/6279f714-9832-411c-af44-17d61e75466c/714123DEST_PATH_RE-S.png)
![Synthesis method of imidazo[1,2-alpha]pyridyl-3-formic acid Synthesis method of imidazo[1,2-alpha]pyridyl-3-formic acid](https://images-eureka.patsnap.com/patent_img/6c5326a8-765a-4e51-9c45-6ba25beb8c9e/201410212487X100002DEST_PATH_IMAGE001.PNG)