Preparation for medicinal intermediate 6-chloro-5-fluroindole for synthesizing anti-cancer medicament
A technology for anticancer drugs and intermediates, which is applied in the field of preparation of pharmaceutical intermediates containing fluorine-containing indole, can solve the problems of large pollution, complicated post-processing, low yield and the like, and achieves simple operation, environmental friendliness and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
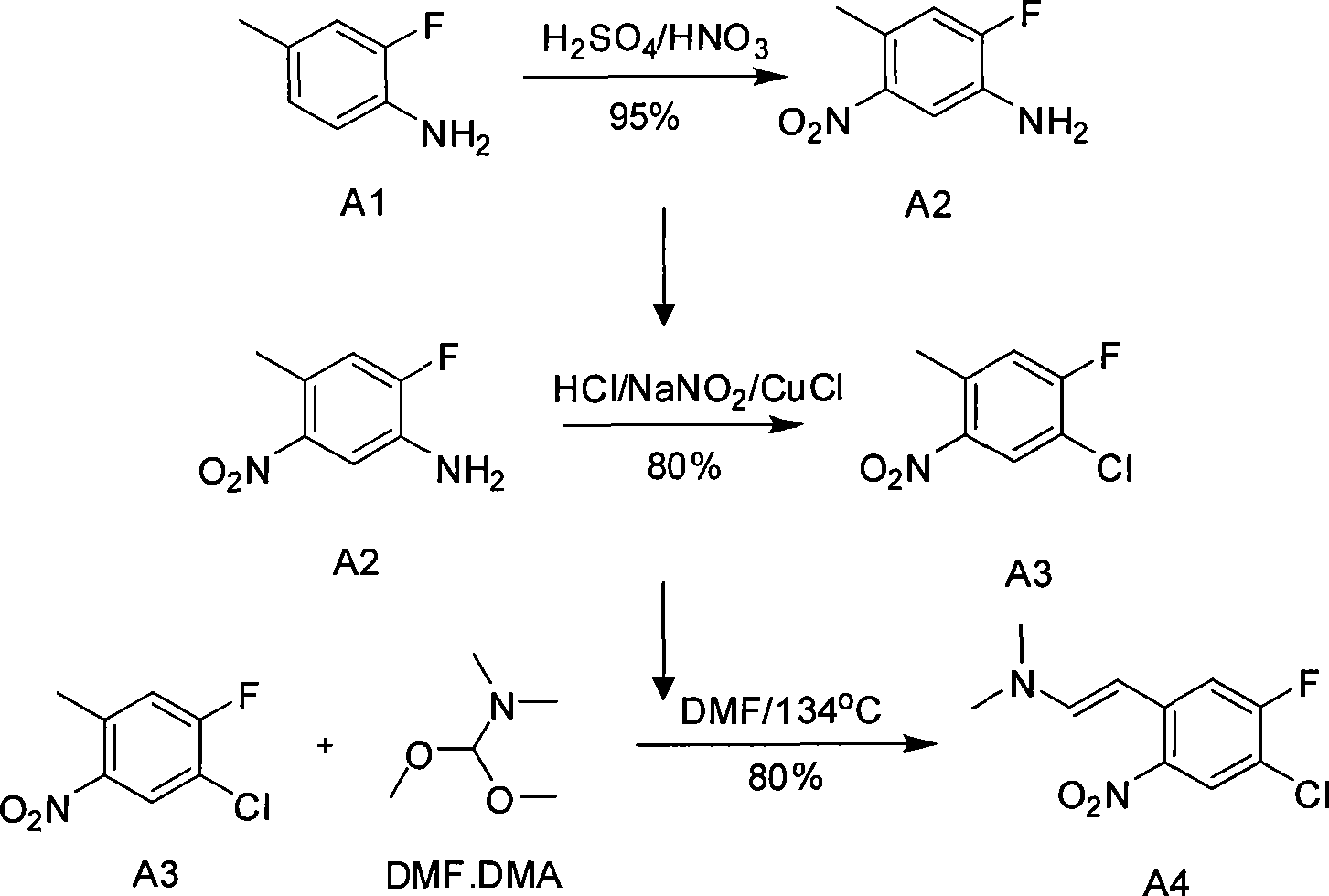
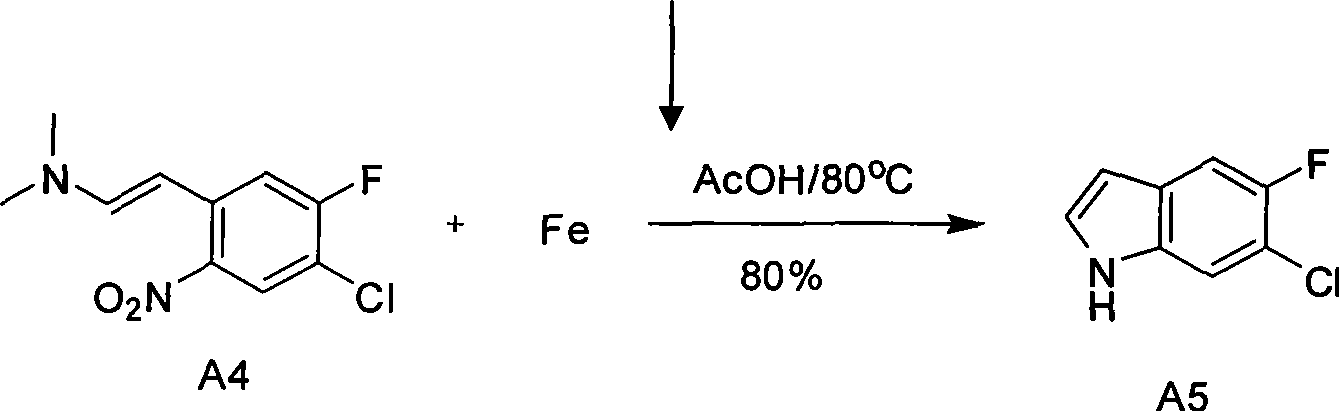
[0025] The first step reaction: Preparation of A2 (3-fluoro-4-amino-6-nitrotoluene)
[0026] In a 10-liter mechanically stirred glass reactor, add 6 liters of concentrated 98% concentrated sulfuric acid at ℃, add 1 kg of A1 dropwise, and finish adding dropwise in 1 hour. After the reaction mixture is dissolved and clarified, the temperature of the reaction system is lowered to minus 10℃ , keep the temperature minus 10°C-minus 5°C, add 760 grams of 65% concentrated nitric acid dropwise, dropwise for 3 hours, then stir for 30 minutes; pour the reaction solution into 10 kg of crushed ice and stir until the ice is completely melted Finally, adjust the pH value of the reaction solution to 7 with aqueous sodium hydroxide solution, keep the temperature below 30°C during adjustment, continue to stir for 30 minutes, filter through a centrifuge device equipped with a filter cloth, and dry to obtain A2 (3-fluoro- 4-amino-6-nitrotoluene) crude product, then washed twice with 10 liters of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com