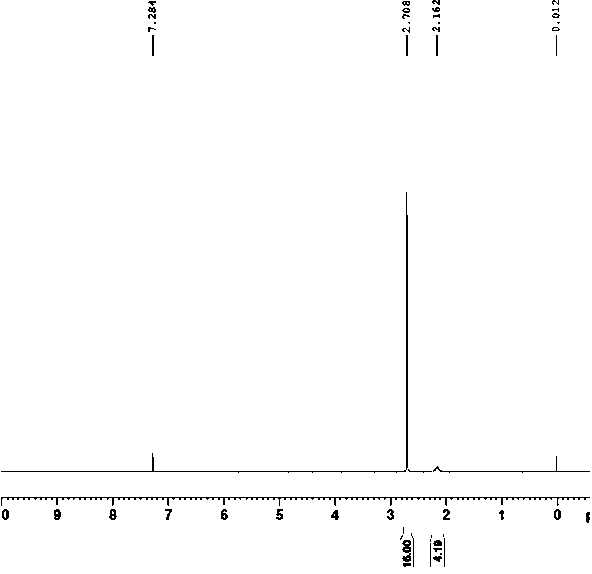
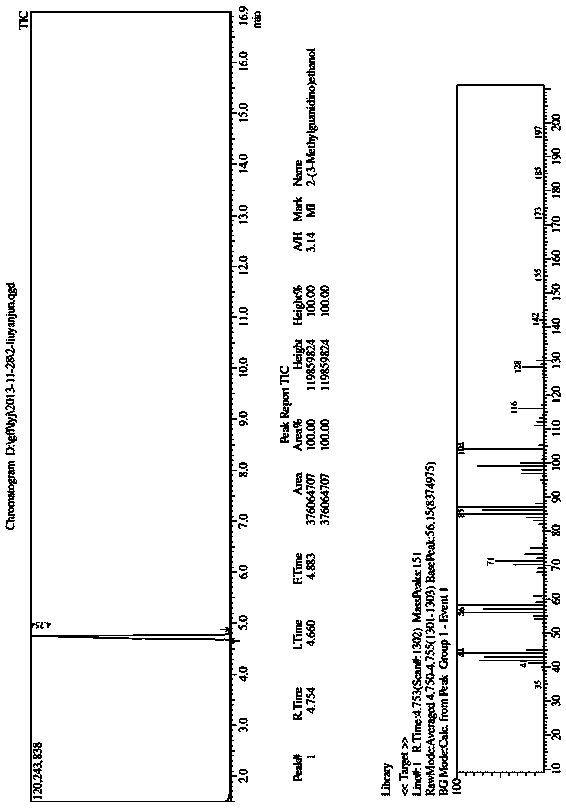
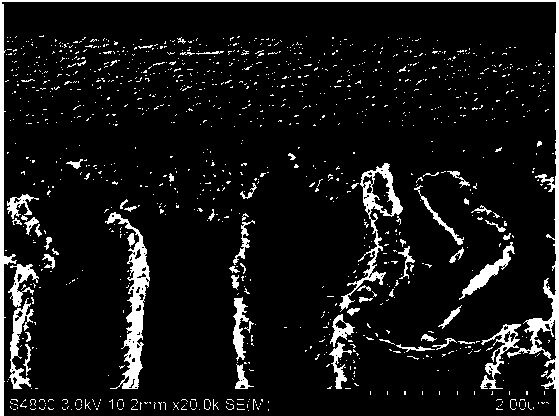Preparation methods of 1,4,7,10-tetraazacyclododecane and nanofiltration membrane
A technology for tetraazacyclododecane, which is applied in the preparation of polyamide nanofiltration membranes and 1,4,7,10-tetraazacyclododecane, can solve complex processes, yield and purity low problems, to achieve the effect of high purity, good retention effect and good oxidation resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) In a three-neck flask equipped with a stirrer and a thermometer, add 86g of TETA, 130mL of solvent toluene, and 20g of water, stir, and cool to below 10°C, white crystals are precipitated, filtered by suction, and dried in a vacuum desiccator to obtain White crystals, 98.1 g of TETA hydrated crystals.
[0027] (2) Add 98.1g of TETA hydrated crystals into a three-necked flask with a water separator, add an appropriate amount of toluene, and heat to reflux to separate water. When the temperature of the liquid rose to 122°C, the water separation was stopped, and the TETA toluene solution in the reaction bottle was dried with anhydrous potassium carbonate. After standing still for 5 hours (so that potassium carbonate can fully absorb water and precipitate), filter potassium carbonate, evaporate toluene under reduced pressure, and obtain 58.9 g of linear TETA.
[0028] (3) Add TETA58.9g, DMF-DMA96.09g, and solvent toluene 200mL into a four-necked flask equipped with a r...
Embodiment 2
[0032] (1) In a three-neck flask equipped with a stirrer and a thermometer, add 64.8g of TETA, 98mL of solvent toluene, and 15.1g of water, stir, and cool to below 10°C, white crystals are precipitated, filtered by suction, and dried in a vacuum desiccator , to obtain white crystals, that is, 81.3 g of TETA hydrated crystals.
[0033] (2) Add 81.3g of TETA hydrated crystals into a three-necked flask with a water separator, add an appropriate amount of toluene, and heat to reflux to separate water. When the temperature of the liquid rose to 123°C, the water separation was stopped, and the TETA toluene solution in the reaction bottle was dried with anhydrous potassium carbonate. After standing still for 4.5h, potassium carbonate was filtered, and the toluene was evaporated under reduced pressure to obtain 50.2g of linear TETA.
[0034] (3) In a four-necked flask equipped with a reflux condenser, a thermometer, and a stirrer, add 50.2g of TETA, 80.7g of DMF-DMA, and 195mL of tol...
Embodiment 3
[0038] (1) In a three-necked flask equipped with a stirrer and a thermometer, add 110g of TETA, 166mL of solvent toluene, and 31.3g of water, stir, and cool to below 10°C, white crystals are precipitated, filtered by suction, and dried in a vacuum desiccator. Obtained white crystals, namely 129 g of TETA hydrated crystals.
[0039] (2) Add 129g of TETA hydrated crystals into a three-necked flask with a water separator, add an appropriate amount of toluene, and heat to reflux to separate water. When the temperature of the liquid rose to 125°C, the water separation was stopped, and the TETA toluene solution in the reaction bottle was dried with anhydrous potassium carbonate. After standing for 5 hours, potassium carbonate was filtered, and the toluene was evaporated under reduced pressure to obtain 81.9 g of linear TETA.
[0040] (3) Add TETA81.9g, DMF-DMA135.7g, and solvent toluene 300mL into a four-necked flask equipped with a reflux condenser, a thermometer, and a stirrer. U...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com