Patents
Literature
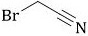
33 results about "Bromoacetonitrile" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
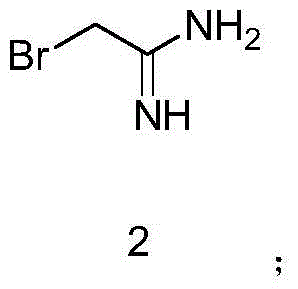
Packaging 5, 25, 100 g in glass bottle Application Bromoacetonitrile was used in the synthesis of 1-cyanomethyl-1,1-dimethy lhydrazinium bromide.
Synthesis method of 6-chloroimidazo[1,2-a]pyridine-3-formonitrile
InactiveCN103896941AMild reaction conditionsEasy to operateOrganic chemistryN dimethylformamideSynthesis methods
The invention relates to a synthesis method of 6-chloroimidazo[1,2-a]pyridine-3-formonitrile. The synthesis method of the 6-chloroimidazo[1,2-a]pyridine-3-formonitrile comprises the following steps: reacting 2-amino-5-chloropyridine and N,N-dimethylformamide dimethylacetal at a temperature ranging from 50 to 110 DEG C for 2-10 hours to obtain (E)-N'-(5-chloropyridine-2-yl)-N,N-dimethylformamidine, next, reacting (E)-N'-(5-chloropyridine-2-yl)-N,N-dimethylformamidine with bromoacetonitrile at a temperature ranging from 50 to 150 DEG C for 5-35 hours under the action of a base in a solvent, then carrying out ethyl acetate extraction, water washing, drying and rotary evaporation and concentration to obtain the coarse product of the 6-chloroimidazo[1,2-a]pyridine-3-formonitrile, and recrystallizing the coarse product solvent to obtain the pure product. The synthesis method of the 6-chloroimidazo[1,2-a]pyridine-3-formonitrile has the beneficial effects of mild reaction conditions, easiness for operation, stable product quality and high product purity.
Owner:SHANDONG YOUBANG BIOCHEM TECH
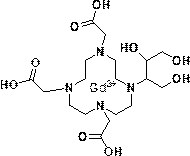
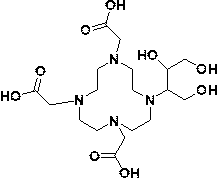
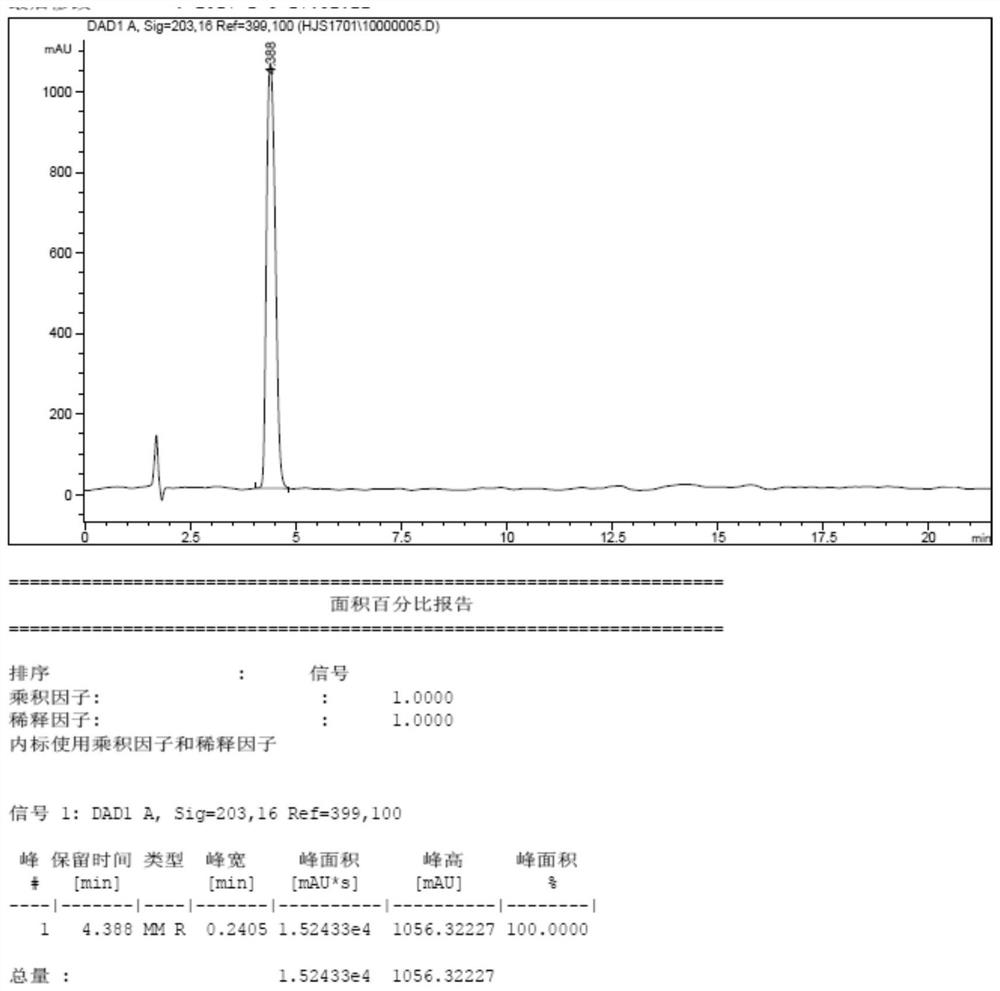
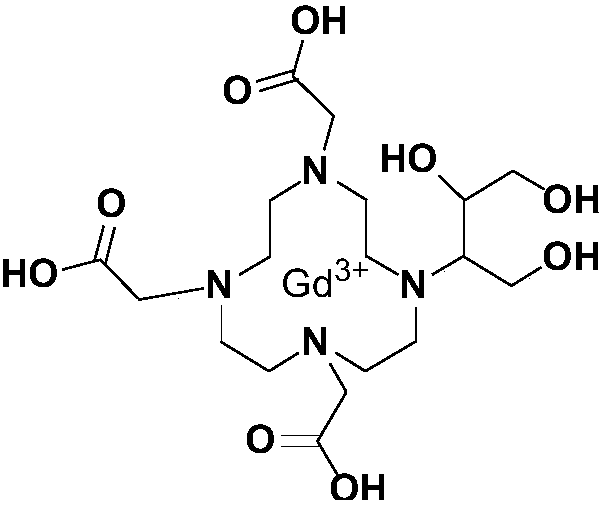
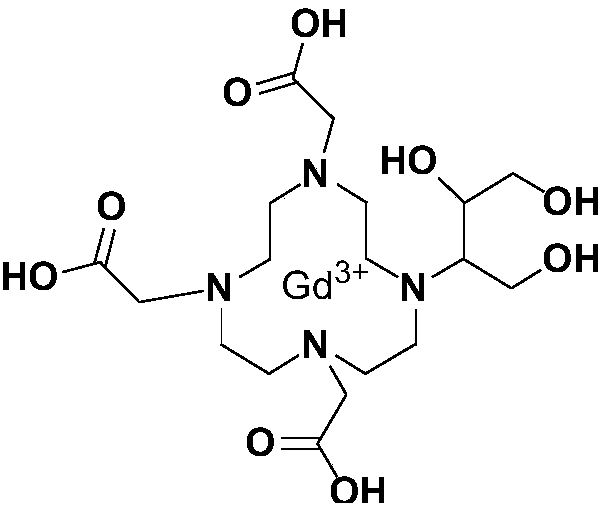
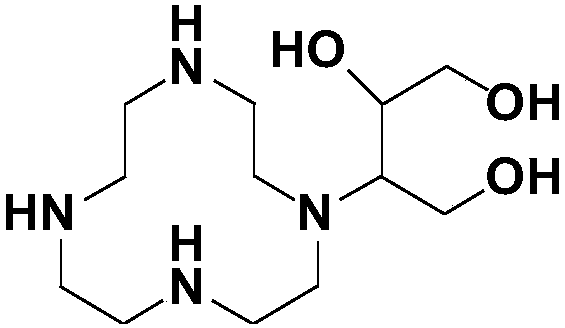
Method for preparing high-purity Gadobutrol
The invention relates to a method for preparing high-purity Gadobutrol. The method uses 3-(1,4,7,10-tetraazacyclododecane-1-yl)butane-1,2,4-triol to react with bromoacetonitrile or bromoacetamide, anobtained intermediate is hydrolyzed under an alkaline condition, and a hydrolysate is complexed with a gadolinium ion source to obtain the high-purity Gadobutrol. The preparation process of the methodis simple and mild, and the method is suitable for large-scale process production.
Owner:CHEN STONE GUANGZHOU CO LTD
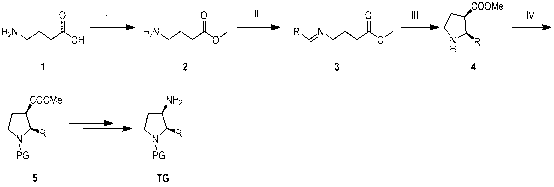
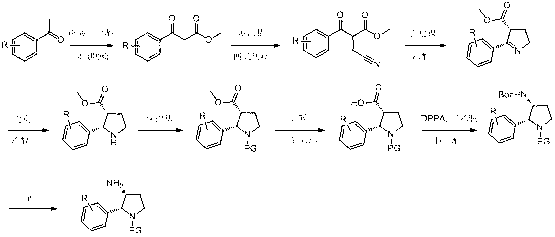
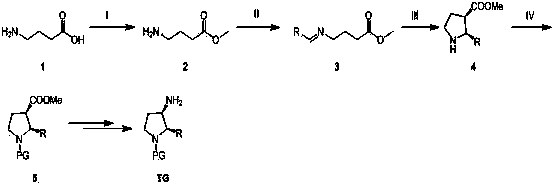
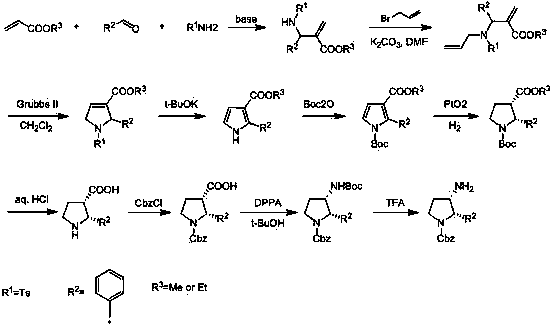
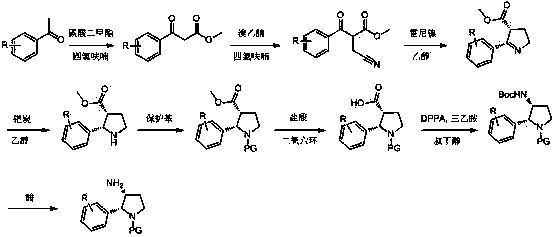
Synthesis method of cis-3-amino-2-arylpyrrolidine derivative
ActiveCN102936218AImprove biological activityReduce usageOrganic chemistryBulk chemical productionEnaminePhenyl group
The invention relates to an industrial preparation method of a cis-3-amino-2-arylpyrrolidine derivative, which mainly solves the technical problems that the synthetic route is long, the yield is low, the cost is high, the obtained cis-product content is low, the post treatment operation is difficult, the intermediate compound is difficult to purify, the reaction conditions are severe and large-scale production can not be realized in the existing cis-3-amino-2-arylpyrrolidine compound synthesis. The preparation method comprises the following steps: under the action of sodium hydride, condensing conventional and readily accessible aryl methyl ketone used as an initial raw material and dimethyl carbonate in a tetrahydrofuran solution, and performing bromoacetonitrile alkylation, Raney nickel hydrogenated cyclization and enamine hydrogenated reduction to obtain (cis)-2-(substituted phenyl)-pyrrolidine-3-carboxylate; and then, adding protective groups, performing acid hydrolysis, rearranging, and removing Boc groups to obtain the target product cis-3-amino-2-arylpyrrolidine derivative. The process provided by the invention is simple, has the advantages of fewer reaction steps, high gross production rate and mild conditions, avoids the use of expensive and dangerous reagents, can realize scale-up production and is easy to realize industrial operation.
Owner:CHANGZHOU HEQUAN PHARMA CO LTD
Synthetic method for tert-butyl 1,10-dioxo-2,7-diazaspiro[4.5]decane-7-carboxylate
InactiveCN109608460AReasonable reaction process designHigh yieldOrganic chemistryCarboxylic saltCatalytic effect
The invention relates to a synthetic method for tert-butyl 1,10-dioxo-2,7-diazaspiro[4.5]decane-7-carboxylate, and mainly solves the technical problem that the tert-butyl 1,10-dioxo-2,7-diazaspiro[4.5]decane-7-carboxylate has no synthetic method suitable for industrialization. The synthetic method comprises the following four steps: step 1, dissolving a compound 1 and bromoacetonitrile into anhydrous acetone, and adding potassium carbonate into the above reaction system to obtain a compound 2; step 2, performing a reaction on the compound 2 under the action of sodium borohydride to obtain a compound 3; step 3, introducing hydrogen, and performing ring closing on the compound 3 under the catalytic effect of nickel to obtain a compound 4; and step 4, performing oxidation on the compound 4 toobtain a compound 5, wherein a reaction formula is shown in the description.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
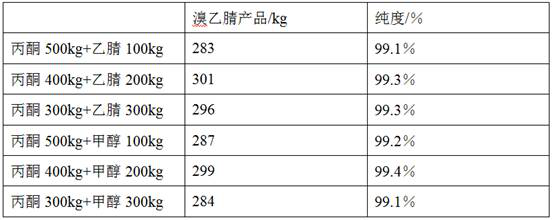
Synthesis method of bromoacetonitrile
PendingCN113582874AAvoid wastingAchieve reuseCarboxylic acid nitrile preparationOrganic compound preparationTetramethylammonium bromideChemical synthesis
The invention belongs to the technical field of chemical synthesis, and particularly relates to a synthesis method of bromoacetonitrile. Tetramethylammonium bromide and chloroacetonitrile are used as raw materials and are easy to transport, store and use, and meanwhile, are high in operability in the use process. Meanwhile, in the reaction process, the formed by-product tetramethylammonium chloride exists in a solid form instead of being dissolved in the used solvent and can be directly removed through filtration. Meanwhile, the by-product can be used or sold as a tetramethylammonium chloride product through simple recrystallization treatment and is widely applied to the electronic industry, and high-value utilization is achieved. Besides, the solvent can be recycled through rectification, so that the solvent in the raw material can be reused, the problems of waste of the raw material, environmental pollution and the like are avoided in the process, and an environment-friendly, safe and benefit-maximized production mode and synthesis process of p-bromoacetonitrile are effectively realized.
Owner:宁夏常晟药业有限公司
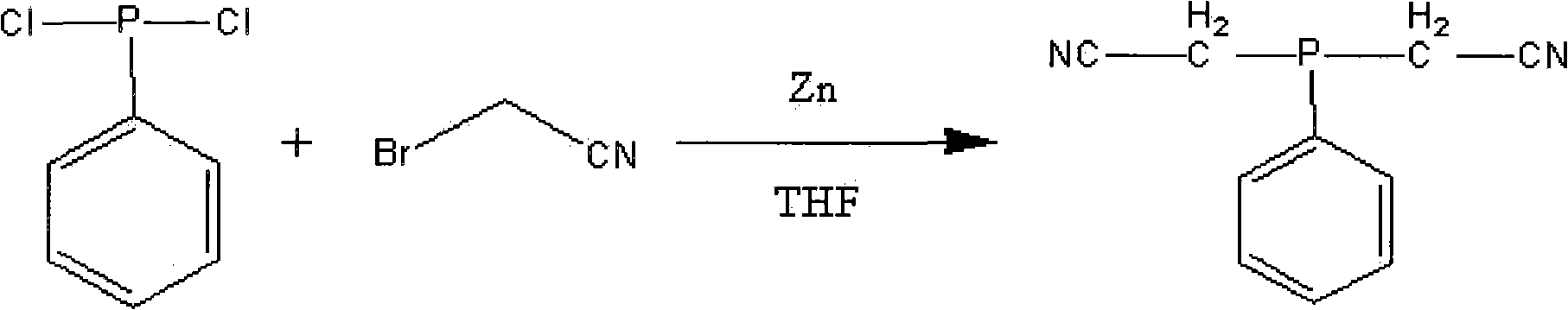
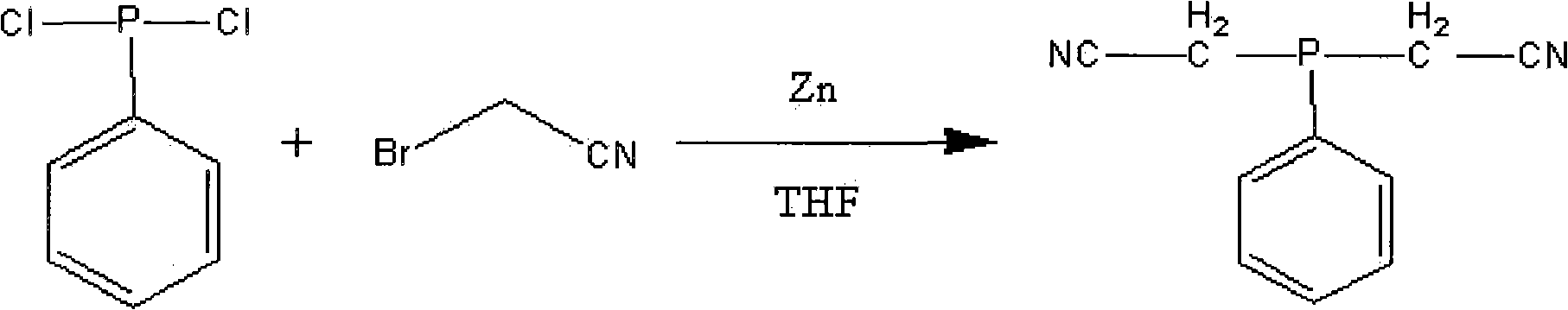
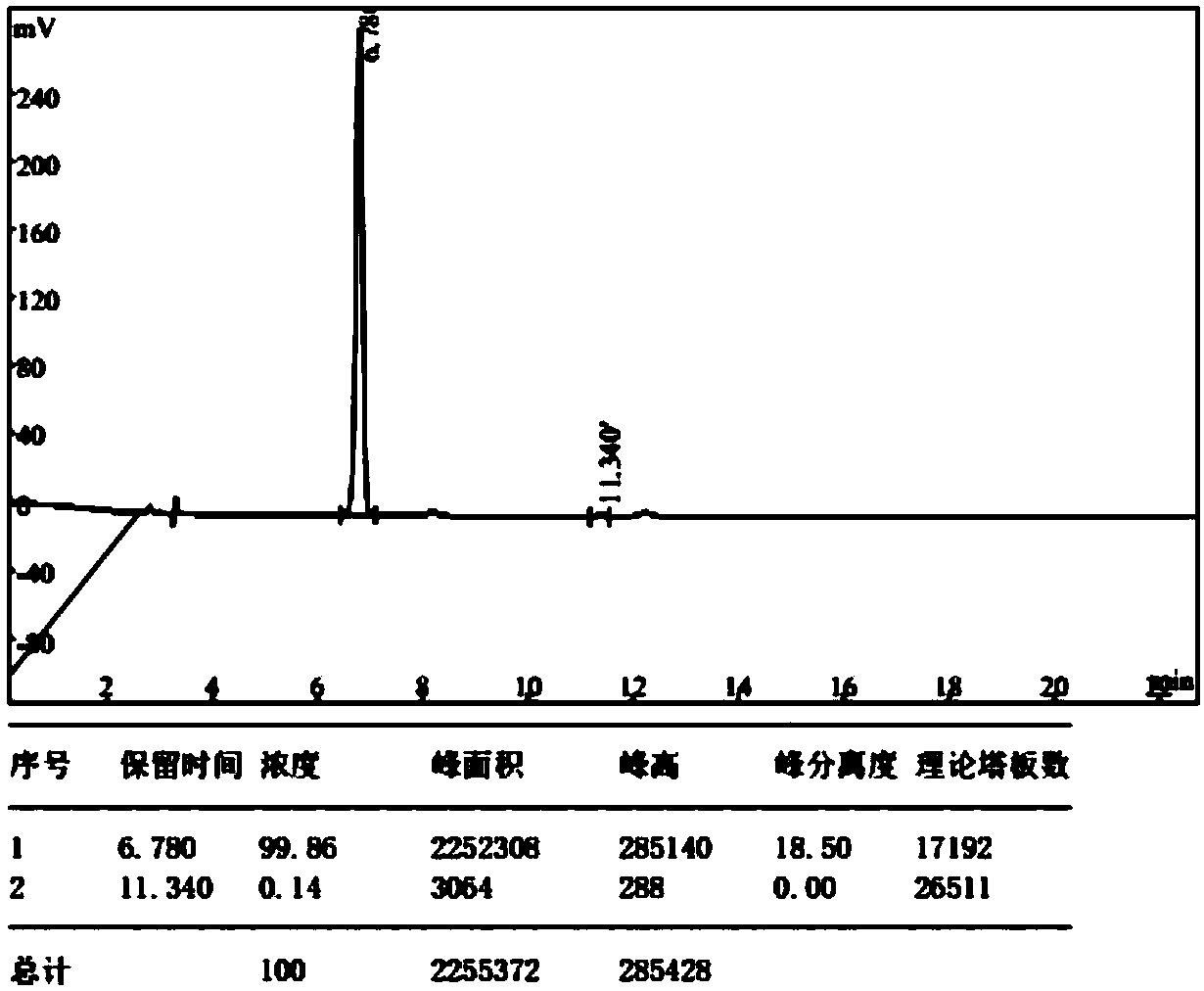
Method for preparing dicyanomethyl phenylphosphine
ActiveCN101974033AHigh purityEasy to produceGroup 5/15 element organic compoundsDichlorophenylphosphineDistillation
The invention discloses a method for preparing dicyanomethyl phenylphosphine, which comprises the following steps of: adding tetrahydrofuran and zinc powder into a reactor, adding dropwise the mixed solution of dichlorophenylphosphine, bromoacetonitrile and the tetrahydrofuran, performing reaction at the constant temperature of 20 to 70 DEG C for 0.5 to 3 hours, performing primary distillation under reduced pressure to recover the tetrahydrofuran and the bromoacetonitrile after the reaction is finished, continuing to add dichloromethane and water into the distilled reactor, performing demixing under stirring to obtain an organic layer, and performing secondary distillation under reduced pressure to remove the dichloromethane to obtain the dicyanomethyl phenylphosphine finished product. The dicyanomethyl phenylphosphine product prepared by the method has the purity of over 98 percent; and the production process is simple, the production is easy to control and the method is favorable for large-scale production.
Owner:SHANDONG UNIV OF SCI & TECH
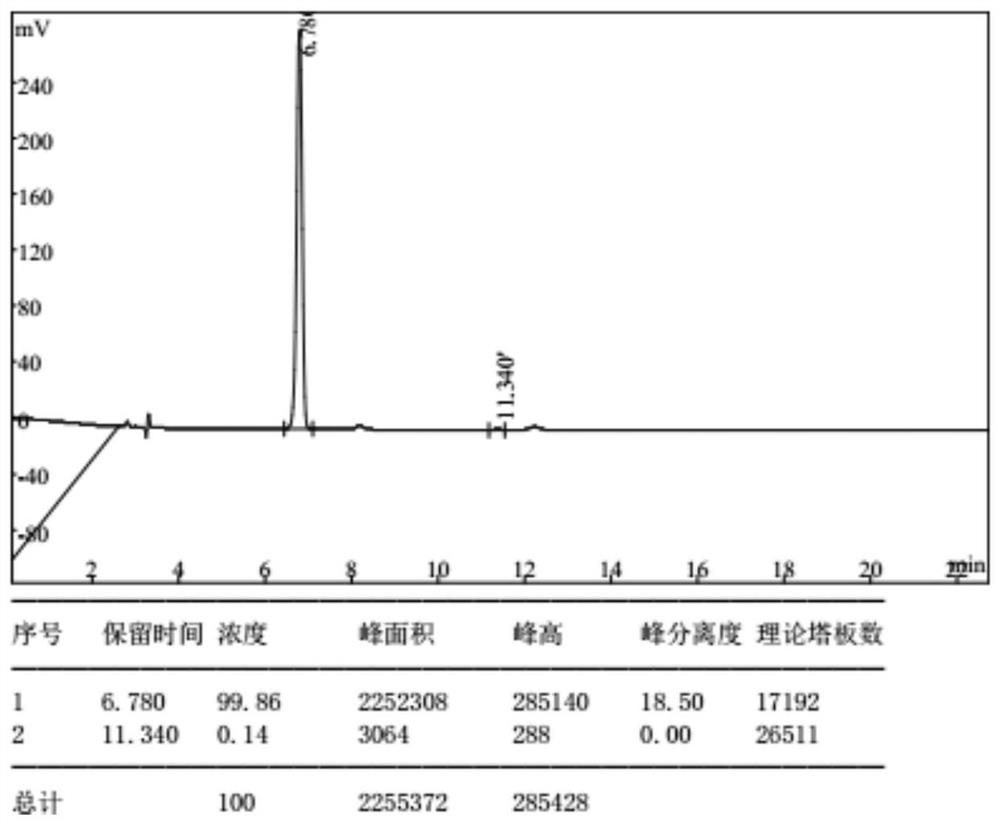
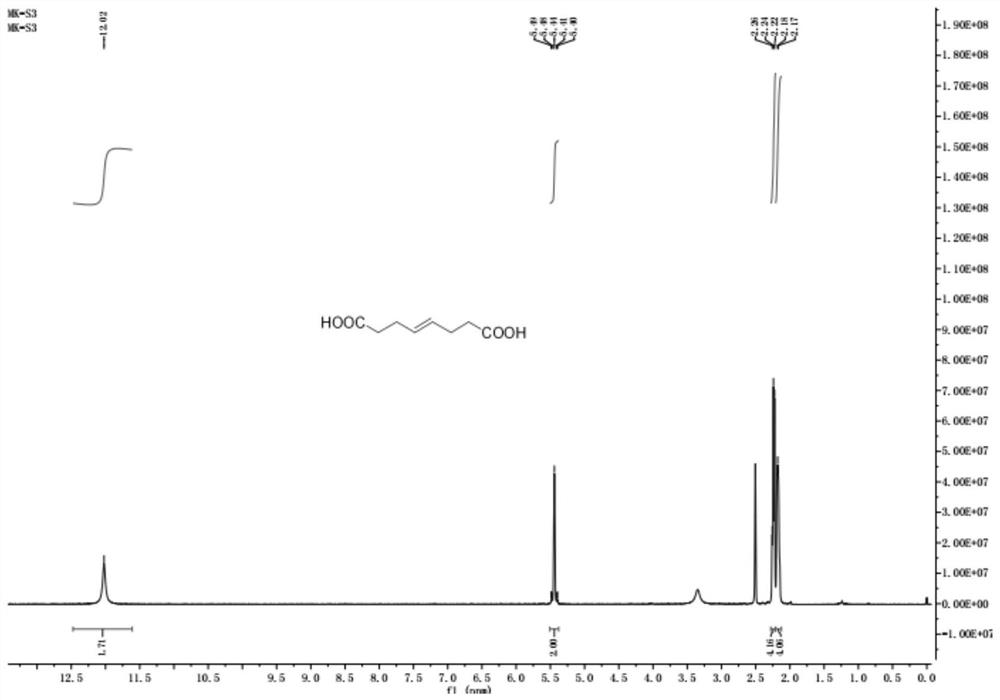
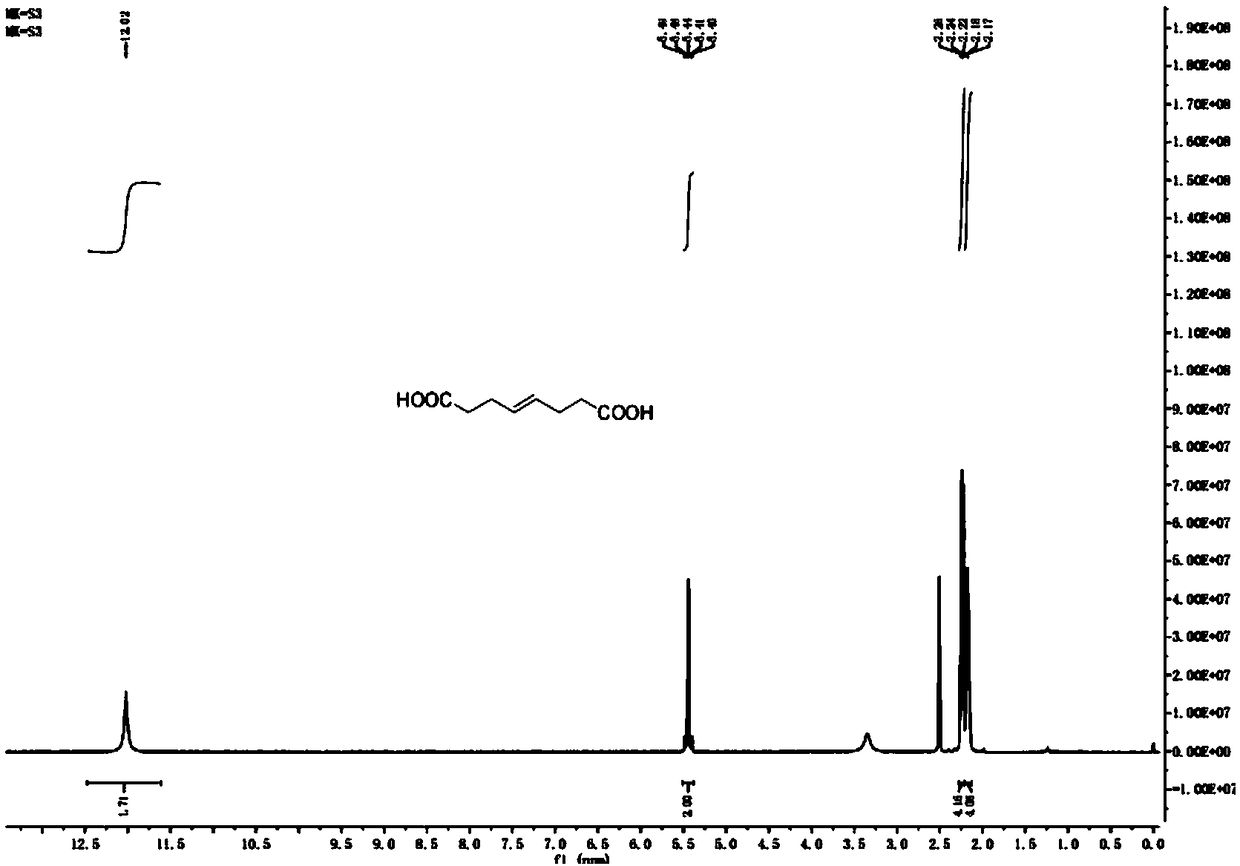
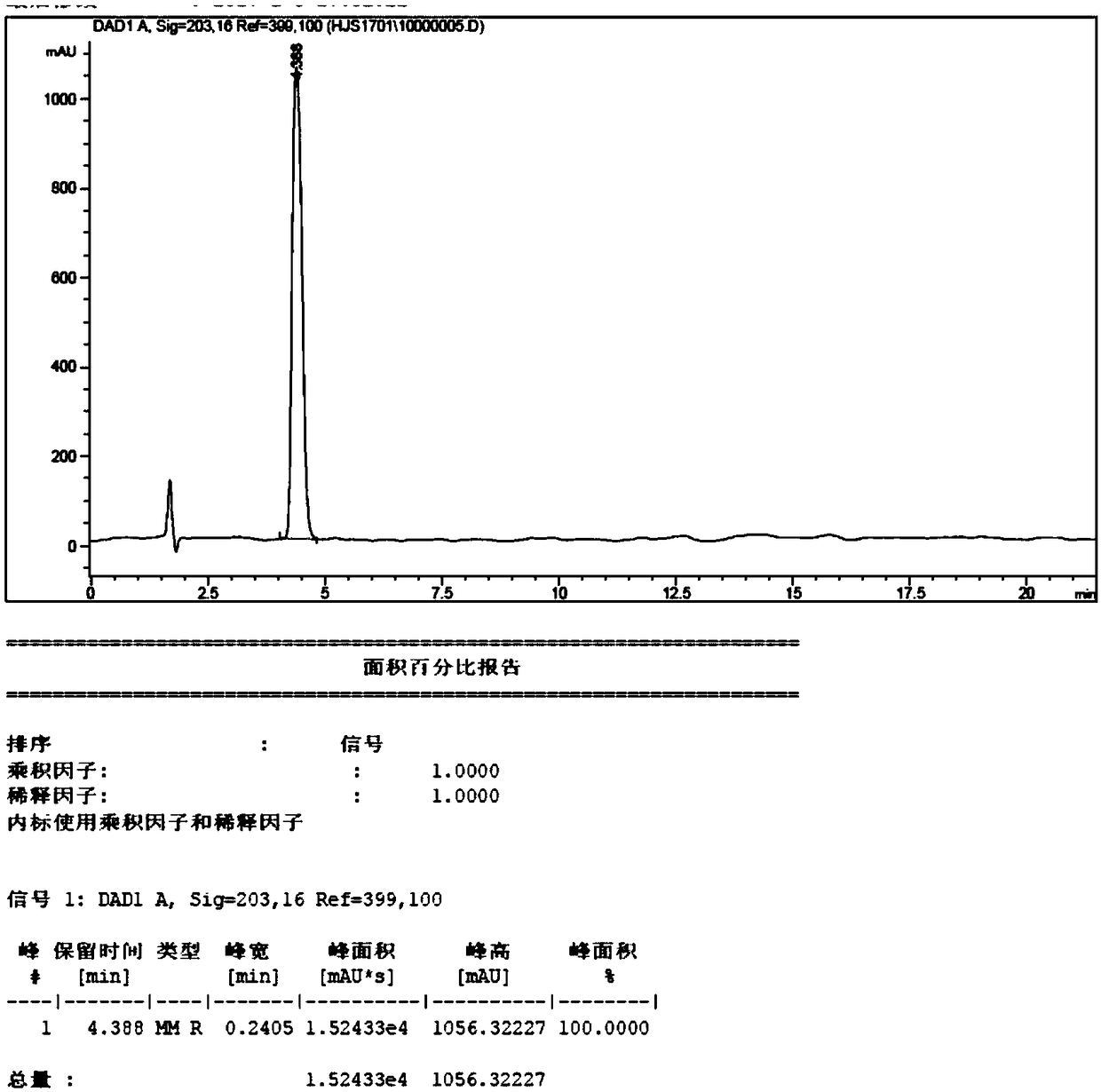
Preparation method of (E)-octyl-4-alkene-1,8-diacid
ActiveCN109232222ARaw materials are easy to getLow priceCarboxylic acid nitrile preparationOrganic compound preparationChemical synthesisBromine
The invention belongs to the chemical synthesis field, and specifically relates to a preparation method of (E)-oct-4-ene-1,8-diacid. The preparation method comprises the following steps: taking bromoacetonitrile as a starting raw material, reacting with a metal element to obtain a metal composite of bromoacetonitrile, and preparing (E)-oct-4-ene-1,8-dinitrile from the metal composite and 1,4-dibromo-2-butene; and hydrolysing (E)-oct-4-ene-1,8-dinitrile to obtain (E)-oct-4-ene-1,8-diacid. According to the method disclosed by the invention, the starting material is cheap and easily available, and a product with purity of 99% or higher can be obtained by simple post-treatment steps such as liquid-separation extraction and concentration after first-step reaction, and simple extraction after hydrolysing cyano alkali into carboxylic acid or hydrolysing cyan acid into ester in the second step. The process reaction and post-treatment operation are simple, and the obtained product is high in quality, so that using requirements can be met without further purification. The preparation method adopts an environment-friendly process, is economical and practical, and is very suitable for industrial production.
Owner:武汉嘉诺康医药技术有限公司
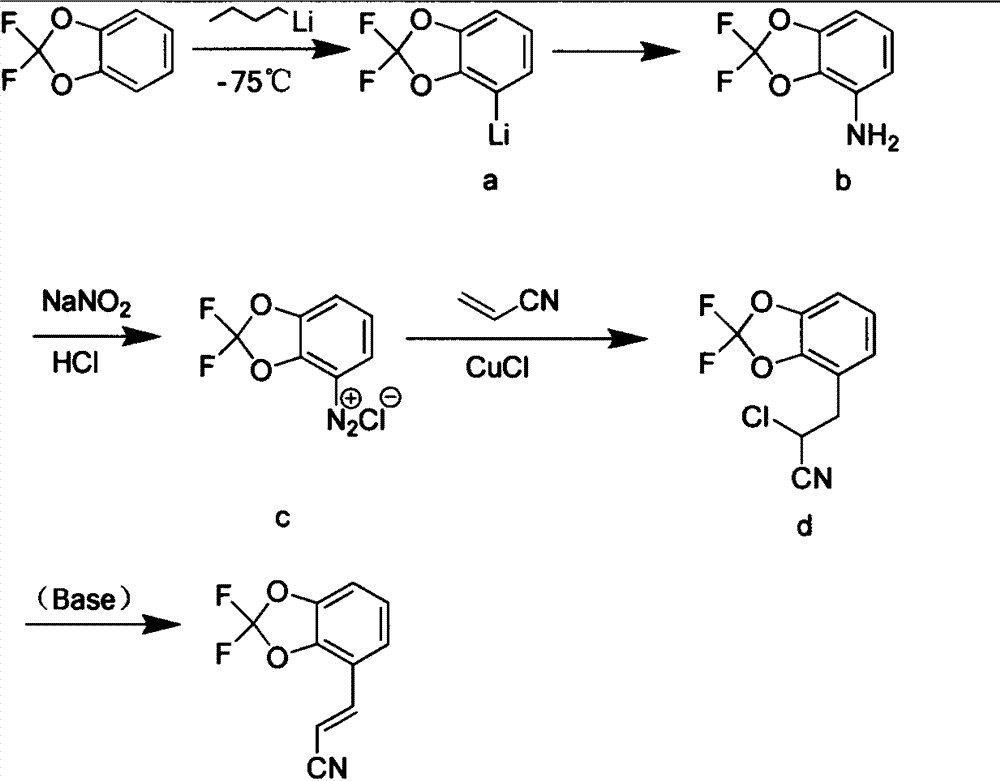
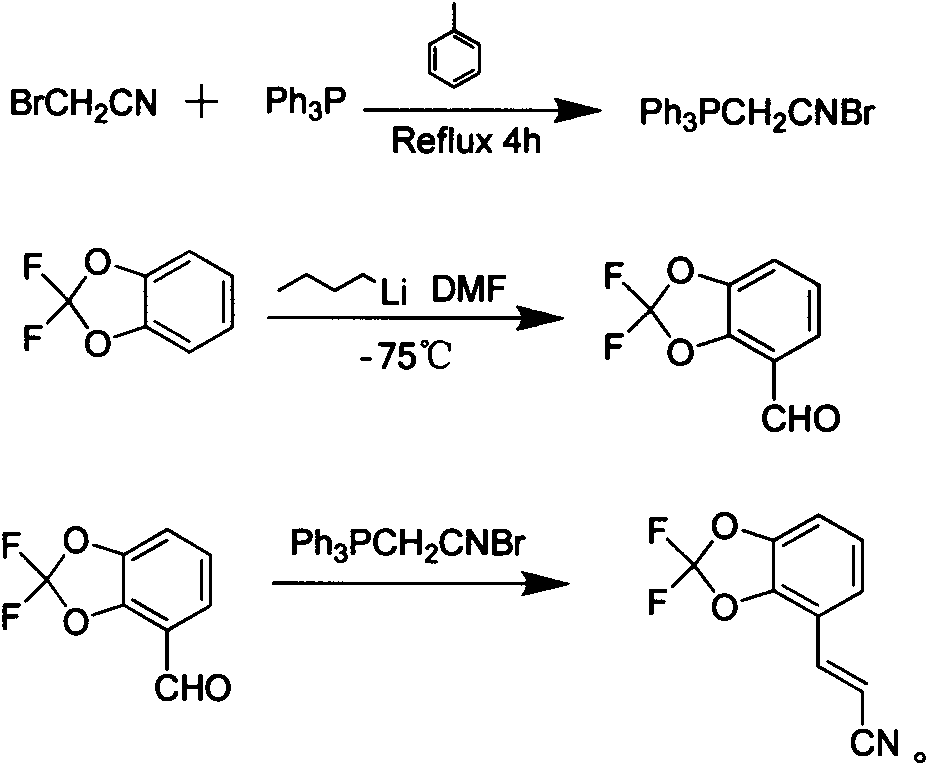
New synthetic method of pesticide fludioxonil intermediate
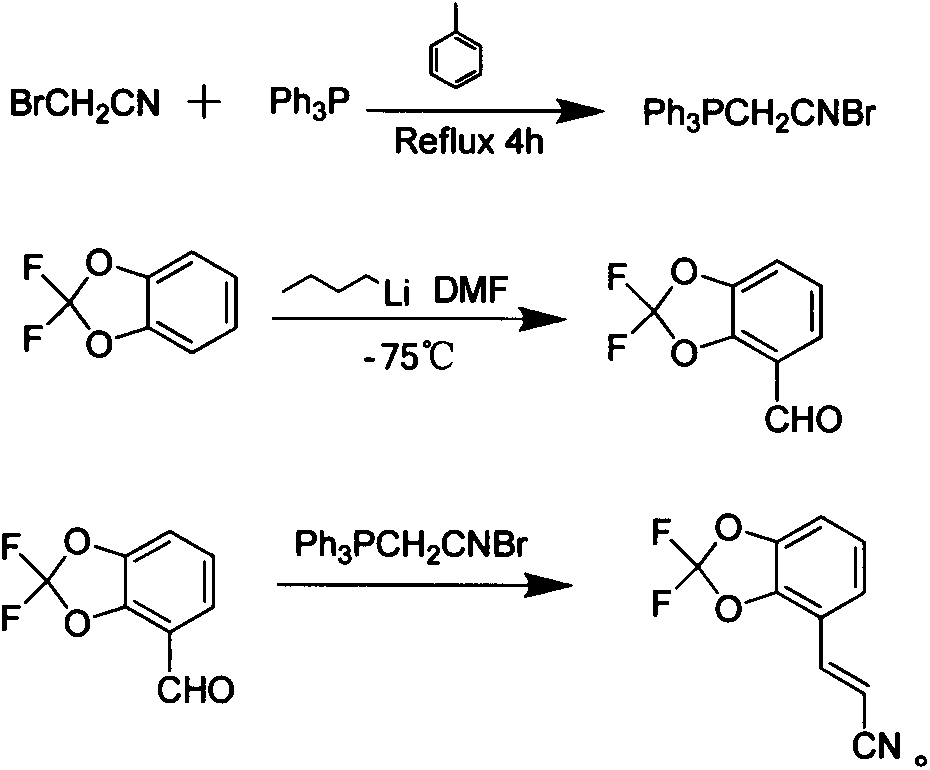
The invention discloses a new synthetic method of a pesticide fludioxonil intermediate. The method comprises the steps of firstly preparing bromoacetonitrile triphenyl phosphate white solid; then preparing 4-formyl-2,2-difluoro-1,3-benzodioxole; finally dissolving 4-formyl-2,2-difluoro-1,3-benzodioxole and bromoacetonitrile triphenyl phosphate into dichloromethane at room temperature, dropping a small amount of a 10% sodium hydroxide solution, and reacting to obtain (E)-3-(2,2-difluoro-1,3-benzodioxolame-4-yl) acrylonitrile. By adopting the method disclosed by the invention, the technical problems that an original synthetic method is more in steps, long in period, relatively highly-toxic in raw materials, serious in three-waste pollution and high in cost are solved.
Owner:SUZHOU VIVOTIDE BIOTECH
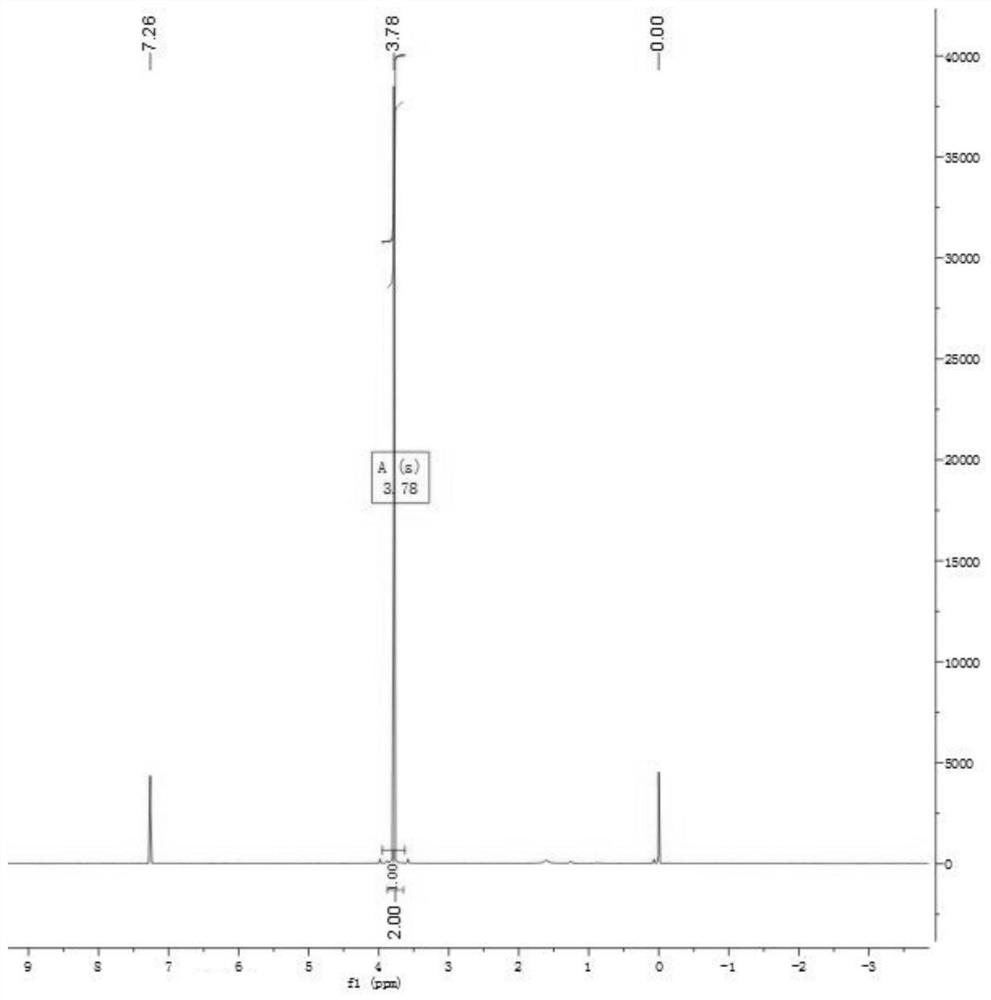
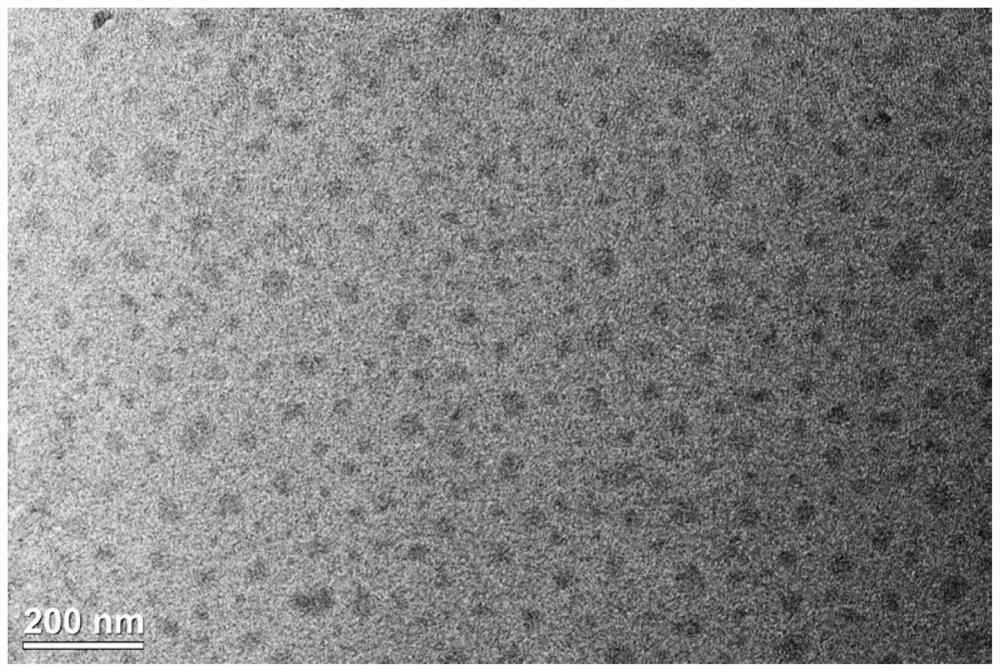
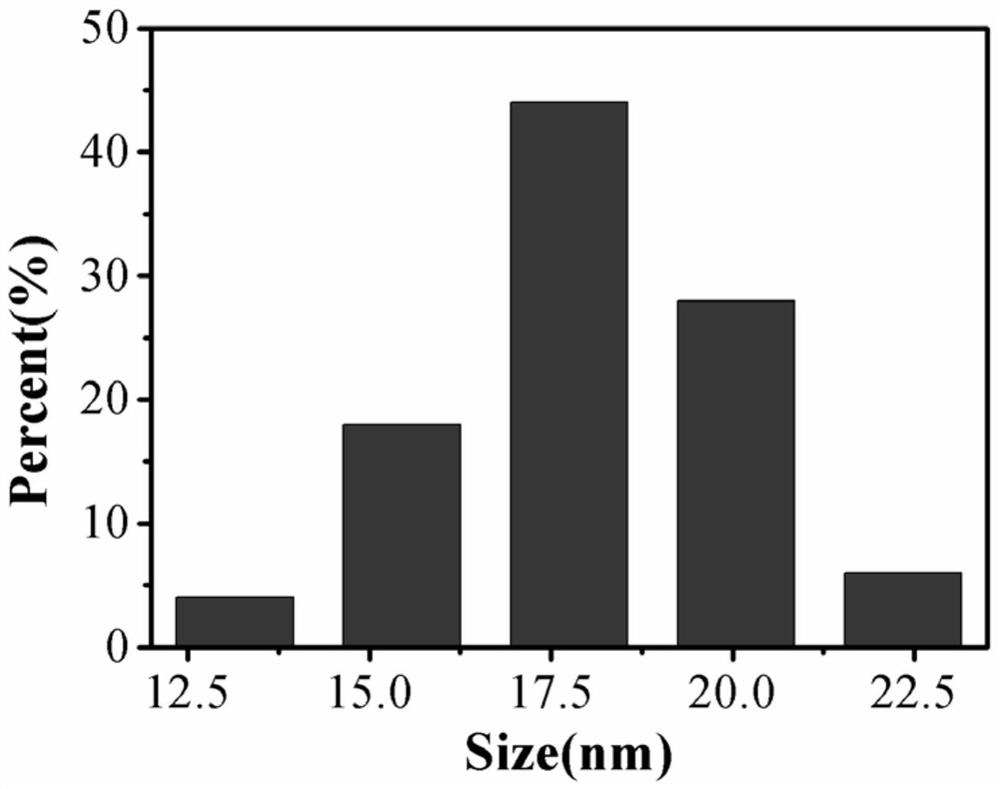
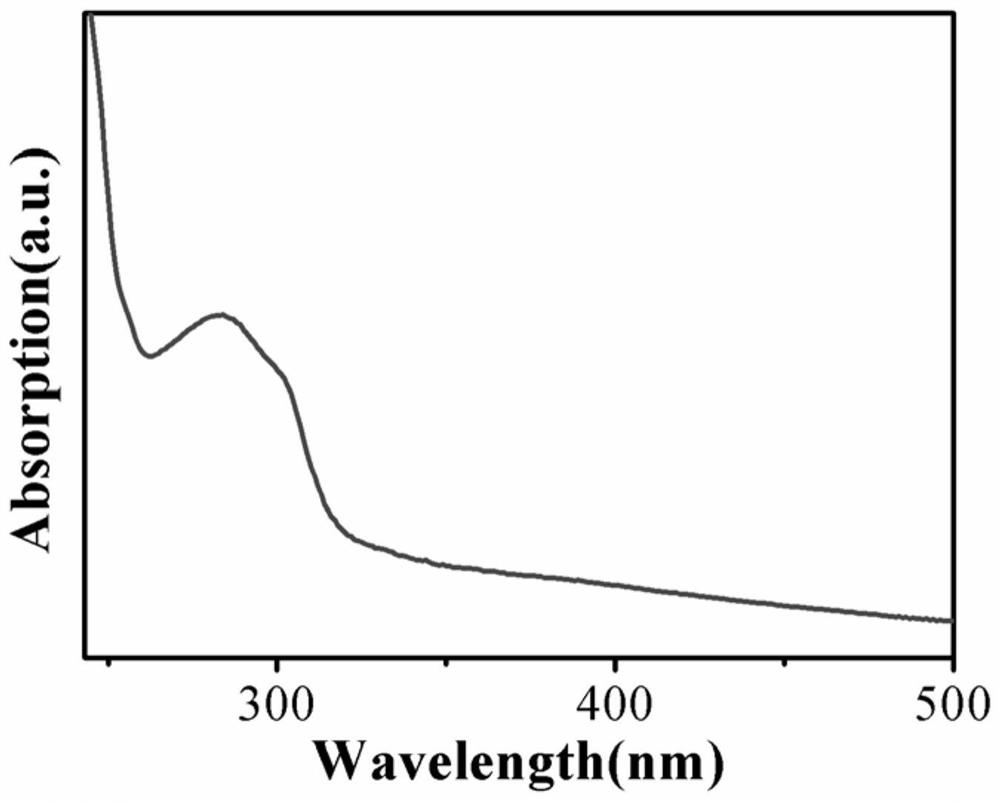
Method and product for preparing carbon dots by using bromoacetonitrile and imidazole compounds
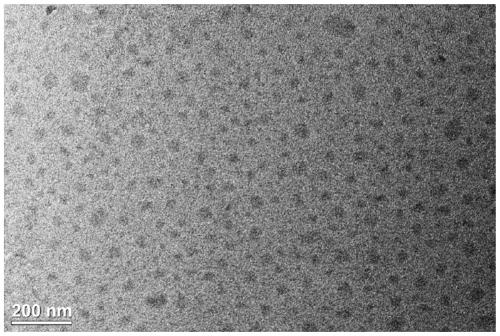
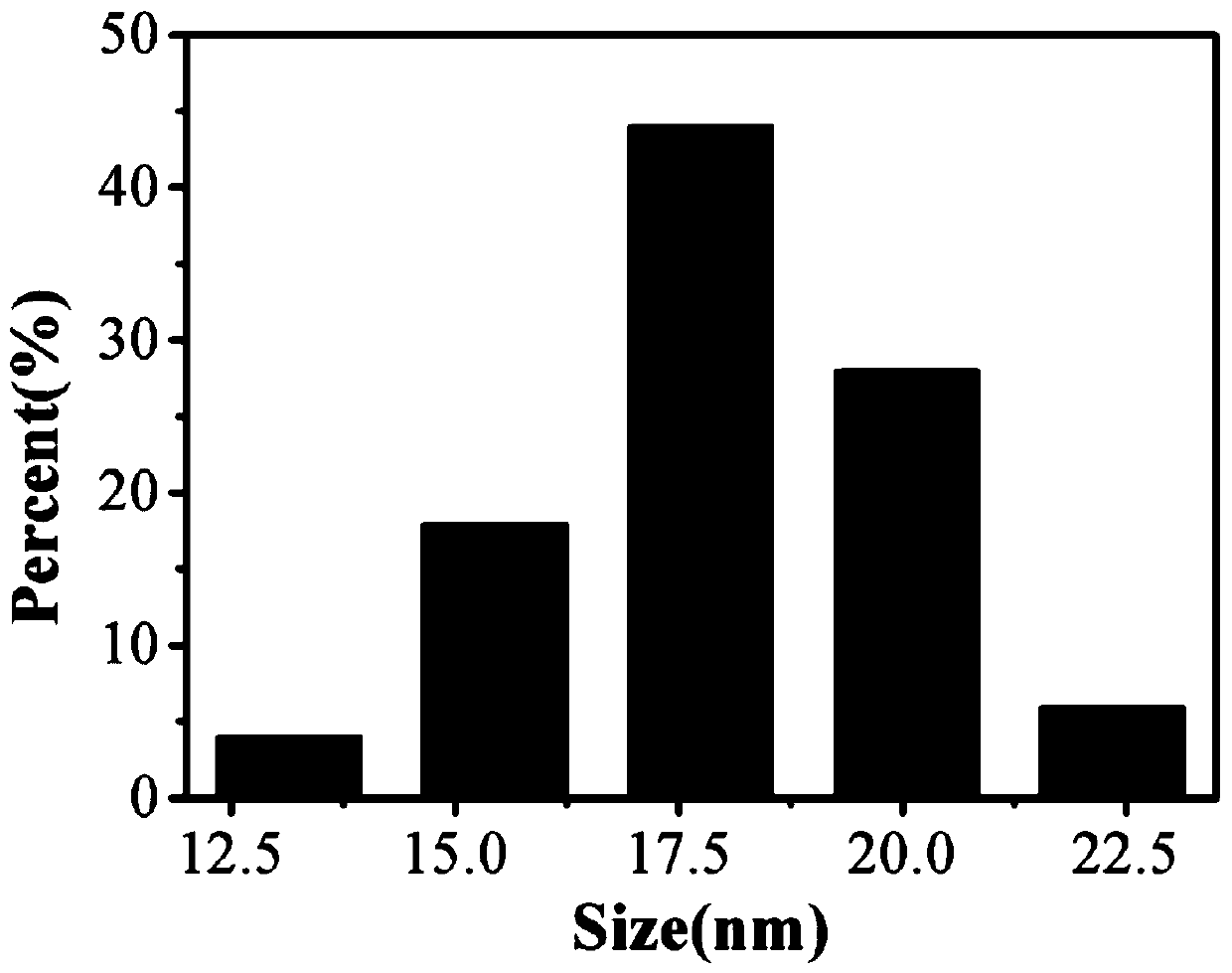
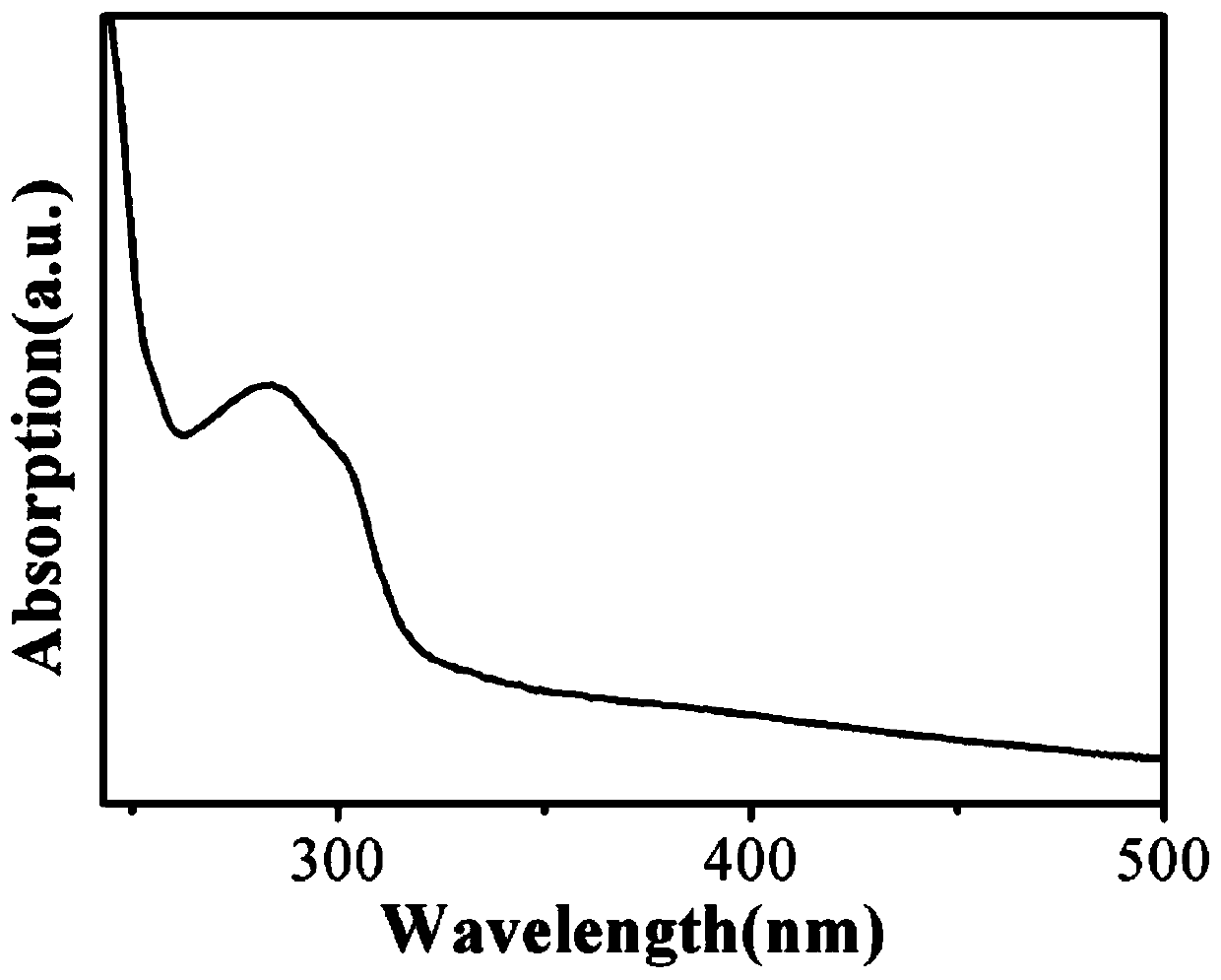
ActiveCN110317606AExcellent fluorescence performanceGood water solubilityNanoopticsLuminescent compositionsBromineAcetonitrile
The invention discloses a method and product for preparing carbon dots by using bromoacetonitrile and imidazole compounds. Specifically, bromoacetonitrile directly reacts with 1-methylimidazole aftermixing to obtain the carbon dots. A synthesis process of the method is simple and green, the synthesized carbon dots have good fluorescence performance and light stability, and a novel method is provided for the synthesis of the carbon dots.
Owner:SOUTHWEST UNIVERSITY
Synthesis method of (E)-4-aryl-3-butenonitrile compound
ActiveCN109651194AImprove economyHigh yieldCarboxylic acid nitrile preparationOrganic compound preparationArylSynthesis methods
The invention belongs to the technical field of organic synthesis chemistry, and discloses a synthesis method of a (E)-4-aryl-3-butenonitrile compound. According to the method, under catalysis of copper iodide, a target compound is synthesized in one step through a coupling reaction of aryl olefins with bromoacetonitrile or iodoacetonitrile. The method has the advantages that the atomic economy ishigh, reaction steps are simple, raw materials are cheap and easy to obtain, the application range of a substrate is wide, and the method is suitable for synthesis of various (E)-4-aryl-3-butenonitrile compounds and suitable for industrial production.
Owner:ANYANG NORMAL UNIV
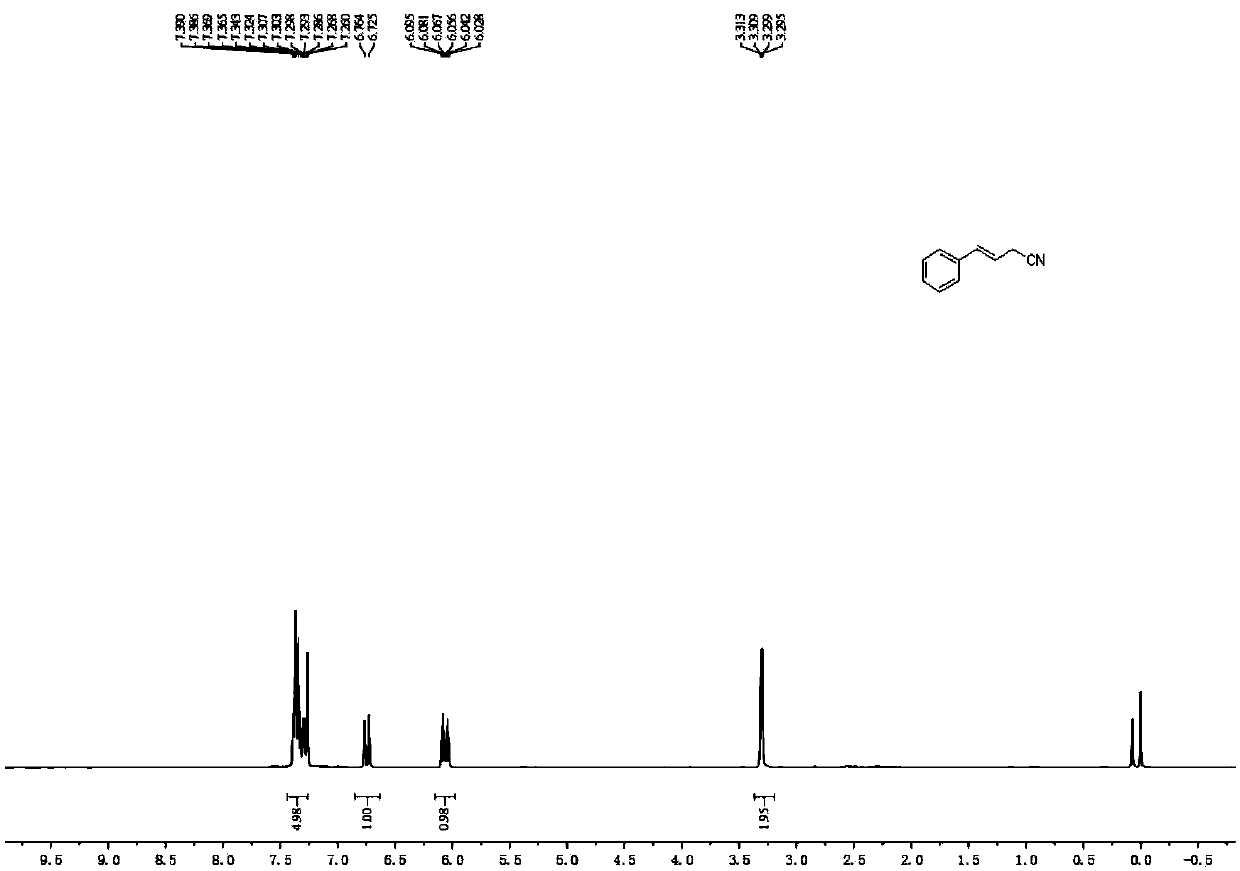
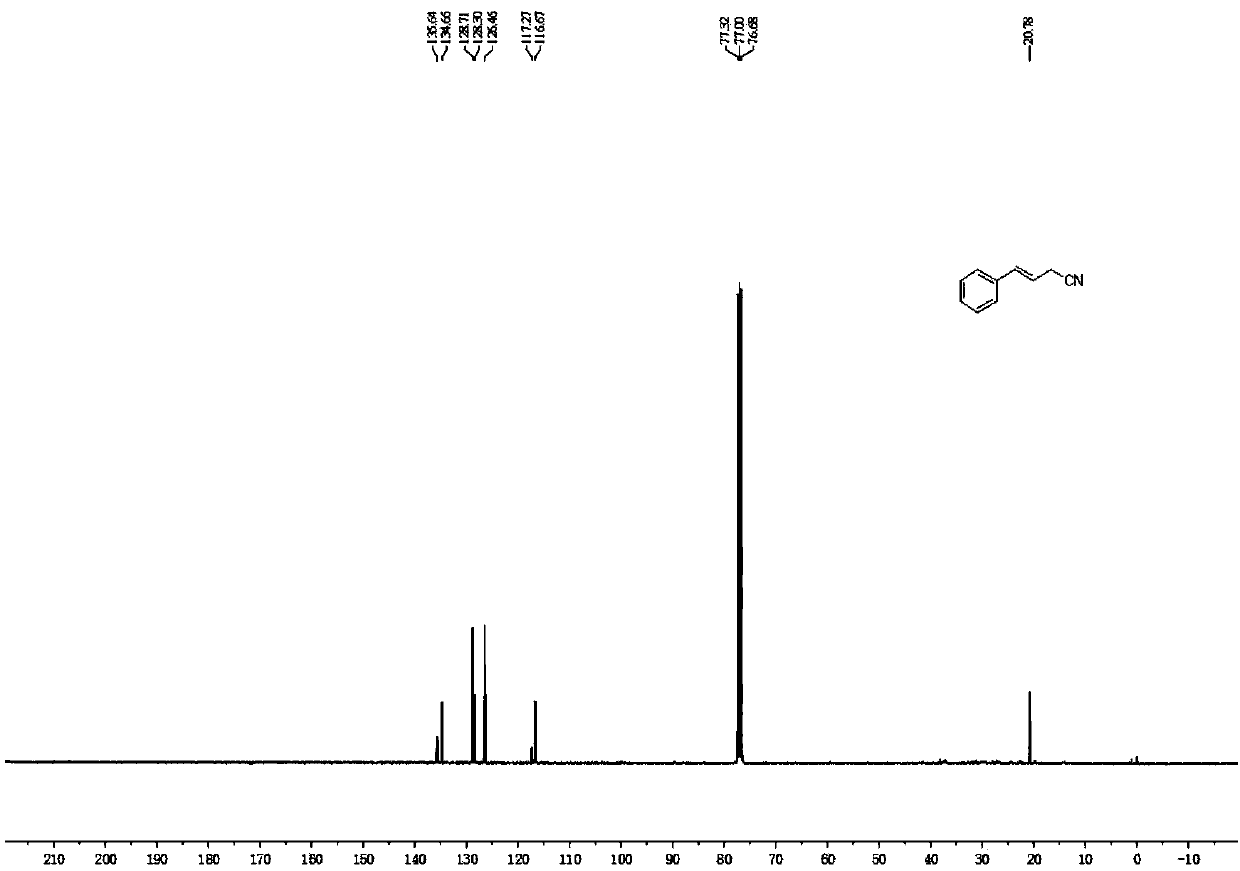
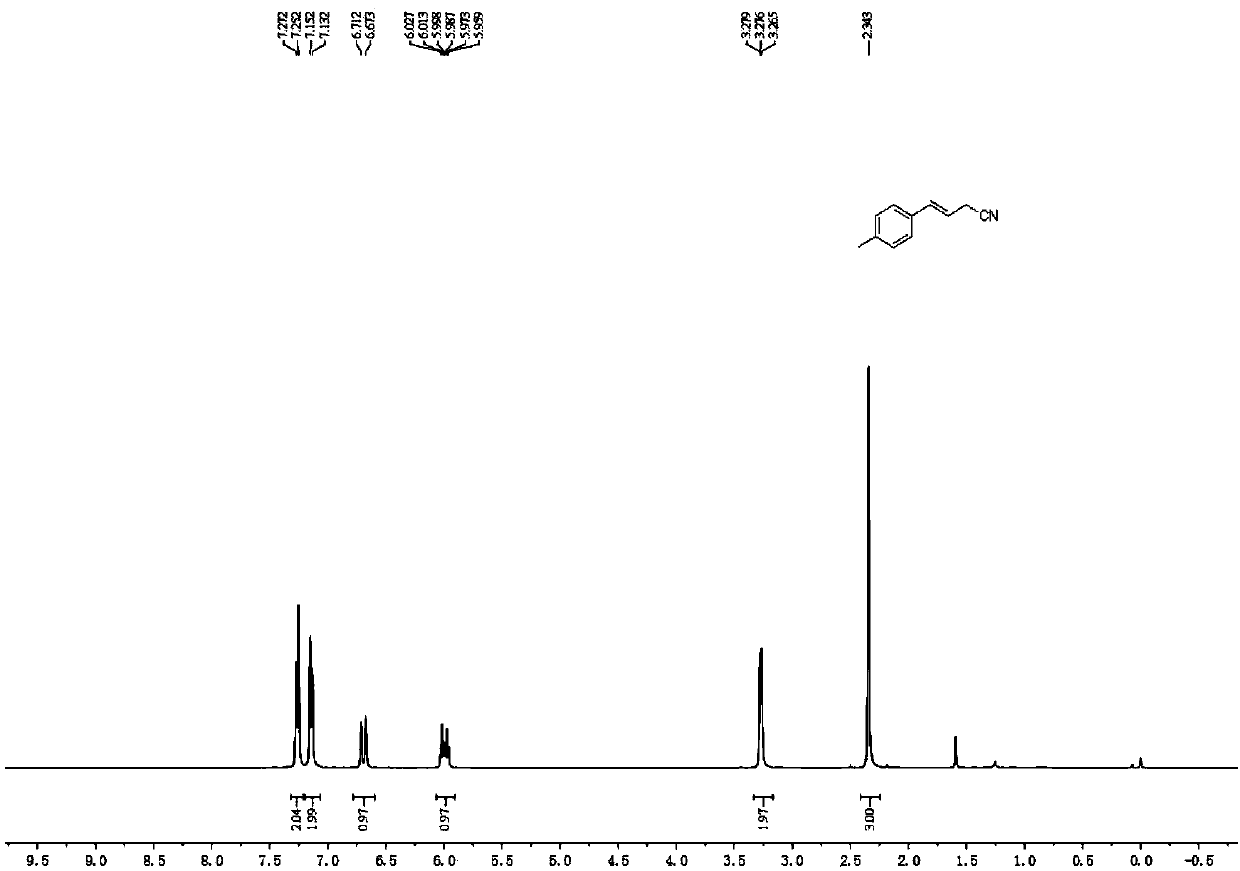
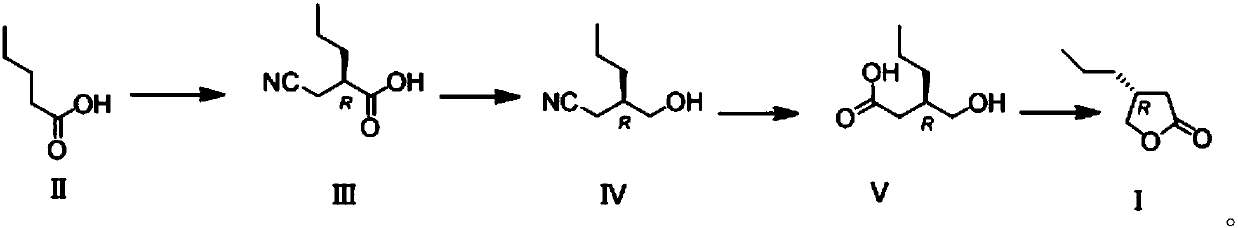
Novel butyrolactone derivative synthesizing method
The invention discloses a novel butyrolactone derivative synthesizing method. The method comprises the following steps: (1) pentanoic acid shown in a formula (II) reacts with bromoacetonitrile after achiral lithiation reaction, and a compound shown in a formula (III) is obtained; (2) a carboxyl group of the compound shown in the formula (III) is reduced by borane, and a compound shown in the formula (IV) is obtained; (3) a cyano group of the compound represented in the formula (IV) is hydrolyzed under the basic condition, a carboxylic acid derivative represented in the formula (V) is obtainedand subjected to a dehydration cyclization reaction, and the butyrolactone derivative represented in the formula (I) is obtained. The method has the advantages that the synthesis cost of raw materialpentanoic acid is low, only four steps are needed, the stereoselectivity is good, and accordingly, the production cost can be obviously reduced. The synthesis route is shown in the description.
Owner:安徽华胜医药科技有限公司
Preparation method of bromoacetonitrile
PendingCN114773225AEmission reductionHigh purityCarboxylic acid nitrile preparationOrganic compound preparationPtru catalystProcess engineering
The invention discloses a bromoacetonitrile preparation method, which comprises: 1) adding chloroacetonitrile, a bromine salt, a catalyst and a solvent to a reactor, and carrying out a reaction at a certain temperature; and 2) after the reaction is finished, cooling the reaction system to room temperature, filtering, washing a filter cake with a small amount of solvent, combining filtrate, distilling the filtrate to recover the solvent, then carrying out reduced pressure rectification on the residual heavy component after the solvent is recovered to obtain the target product bromoacetonitrile, and recovering and reusing the residual catalyst at the bottom of a rectifying still. According to the method disclosed by the invention, chloroacetonitrile and bromine salt are used as raw materials, and bromoacetonitrile is obtained with high yield under mild conditions in the presence of the catalyst. Compared with other routes, the used raw materials are safer and cheaper, and the used process is convenient to operate, free of acid gas and free of corrosion; the obtained product is high in purity and lower in cost. The catalyst used in the process can be recycled, so that the emission of wastes is greatly reduced, and the process route is greener.
Owner:上海巽田科技股份有限公司
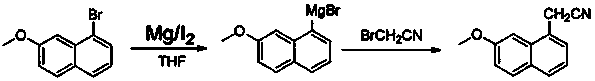
Preparation method of agomelatine intermediate
ActiveCN107353229AReduce turn lossRaw materials are cheap and easy to getCarboxylic acid nitrile preparationOrganic compound preparationOrganic solventGrignard reagent
Owner:XUCHANG HENGSHENG PHARMA
Method for synthetizing 6-bromine imidazo [1, 2-alpha] pyridine-3-carbonitrile
InactiveCN103880843AMild reaction conditionsEasy to operateOrganic chemistryN dimethylformamide2-amino-5-bromopyridine
The invention relates to a method for synthetizing 6-bromine imidazo [1, 2-alpha] pyridine-3-carbonitrile. The method for synthetizing the 6-bromine imidazo [1, 2-alpha] pyridine-3-carbonitrile comprises the following steps: reacting 2-amino-5-bromopyridine with N, N-dimethylformamide dimethyl acetal for 2-10 hours at the temperature of 50-110 DEG C to obtain (E)-N'-(5-bromopyridine-2-yl)-N, N-dimethyl formamidine; in a solvent, reacting (E)-N'-(5-bromopyridine-2-yl)-N, N-dimethyl formamidine with bromoacetonitrile for 5-35 hours at the temperature of 50-150 DEG C under the action of alkali, carrying out ethyl acetate extraction, washing, drying, rotary evaporation and concentration to obtain a crude product of 6- bromine imidazo [1, 2-alpha] pyridine-3-carbonitrile, recrystallizing the crude product by using a solvent to obtain a pure product. The method disclosed by the invention has the beneficial effects that the reaction is mild in condition and easy to operate and the product is stable in quality and high in purity.
Owner:SHANDONG YOUBANG BIOCHEM TECH
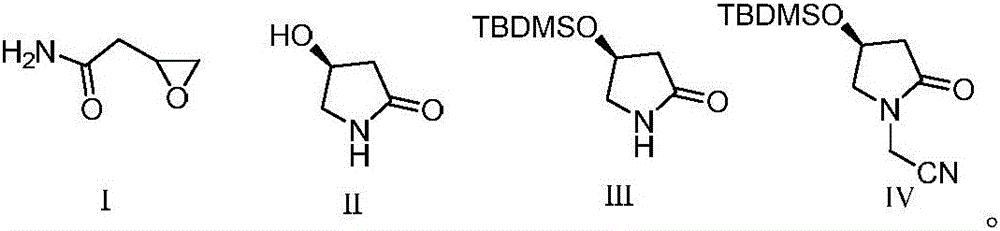
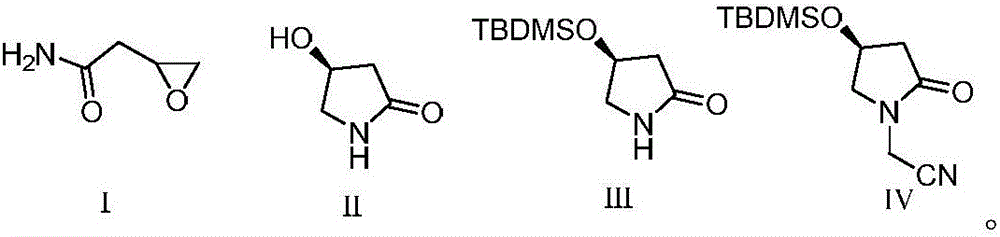
Preparation method of (S)-oxiracetam
The invention discloses a preparation method of (S)-oxiracetam. The preparation method is characterized by comprising the following steps that 1, 2-epoxy ethylacetamide is cyclized in molecules in the presence of a catalyst and alkali to obtain a compound (S)-3-hydroxyl-butyrolactam shown as a formula II; 2, the (S)-3-hydroxyl-butyrolactam obtained in the step 1 reacts with tertiary butyl methyl chlorosilane to obtain a compound protected by the tertiary butyl methyl chlorosilane and shown as a formula III; 3, the compound shown as the formula III performs nucleophilic reaction with bromoacetonitrile under the alkaline condition to obtain a N-cyanomethide shown as a formula IV; 4, the N-cyanomethide shown as the formula IV is subjected to hydrolysis under the acid condition to obtain the (S)-oxiracetam (shown in the description). The method adopts conventional non-achiral raw materials and is low in cost, the (S)-oxiracetam is high in yield, an ee value is high, and a new way is provided for preparation of the (S)-oxiracetam.
Owner:南京帝昌医药科技有限公司
8-acetonitrile oxy imidazo [1,2-a] pyridine-3-carbonitrile preparation method
InactiveCN104910156AReaction raw materials are readily availableReasonable priceOrganic chemistryN dimethylformamideDimethyl acetal
The present invention relates to a 8-acetonitrile oxy imidazo [1,2-a] pyridine-3-carbonitrile preparation method. The preparation method comprises the following steps: N, N-dimethylformamide dimethyl acetal is reacted with 2-amino-3-hydroxy pyridine in a certain proportion at 40-100 DEG C to obtain N, N-dimethyl-N '-2-(3-hydroxy-pyridine) yl-formamidine intermediate, under the effect of an alkali, in a certain solvent, the N, N-dimethyl-N '-2-(3-hydroxy-pyridine) yl-formamidine intermediate is reacted with bromoacetonitrile in certain proportion at 50-160 DEG C for 3-15 hours, after the reaction, a 8-acetonitrile oxy imidazo [1,2-a] pyridine-3-carbonitrile crude product is obtained by cooling to room temperature, extracting with ethyl acetate, washing with water and saturated salt water, drying with anhydrous sodium sulfate, rotation evaporation and concentration, and a pure product is obtained by recrystallization of the crude product. Reaction materials are relatively easy to obtain, and are reasonable in prices, reaction conditions are mild, easy to operate, and easy to control, post-processing is simple, product quality is stable, and purity is high.
Owner:SHANDONG YOUBANG BIOCHEM TECH
Method for preparing bromoacetonitrile from chloroacetonitrile
ActiveCN114507157AHigh reaction yieldSimple and fast operationOrganic compound preparationCarboxylic acid nitrile purification/separationPtru catalystBromoacetonitrile
The invention discloses a method for preparing bromoacetonitrile from chloroacetonitrile, and belongs to the field of chemical engineering. The method comprises the following steps: heating and refluxing chloroacetonitrile, inorganic bromide and an iodine-containing catalyst in a solvent, and purifying after the reaction is completed to obtain bromoacetonitrile. The easily available inorganic bromide is used as the bromine source, the used solvent is environment-friendly and recyclable, the reaction yield is greatly improved, the post-treatment method is simple, and the whole reaction route is clean and environment-friendly.
Owner:沧州维智达美制药有限公司
Method and product for preparing carbon dots by using bromoacetonitrile and imidazole compounds
ActiveCN110317606BExcellent fluorescence performanceGood water solubilityNanoopticsLuminescent compositionsCombinatorial chemistryBromoacetonitrile
Owner:SOUTHWEST UNIV
Synthesis method of cis-3-amino-2-arylpyrrolidine derivative
ActiveCN102936218BImprove biological activityReduce usageOrganic chemistryBulk chemical productionEnaminePhenyl group
The invention relates to an industrial preparation method of a cis-3-amino-2-arylpyrrolidine derivative, which mainly solves the technical problems that the synthetic route is long, the yield is low, the cost is high, the obtained cis-product content is low, the post treatment operation is difficult, the intermediate compound is difficult to purify, the reaction conditions are severe and large-scale production can not be realized in the existing cis-3-amino-2-arylpyrrolidine compound synthesis. The preparation method comprises the following steps: under the action of sodium hydride, condensing conventional and readily accessible aryl methyl ketone used as an initial raw material and dimethyl carbonate in a tetrahydrofuran solution, and performing bromoacetonitrile alkylation, Raney nickel hydrogenated cyclization and enamine hydrogenated reduction to obtain (cis)-2-(substituted phenyl)-pyrrolidine-3-carboxylate; and then, adding protective groups, performing acid hydrolysis, rearranging, and removing Boc groups to obtain the target product cis-3-amino-2-arylpyrrolidine derivative. The process provided by the invention is simple, has the advantages of fewer reaction steps, high gross production rate and mild conditions, avoids the use of expensive and dangerous reagents, can realize scale-up production and is easy to realize industrial operation.
Owner:CHANGZHOU HEQUAN PHARMA CO LTD
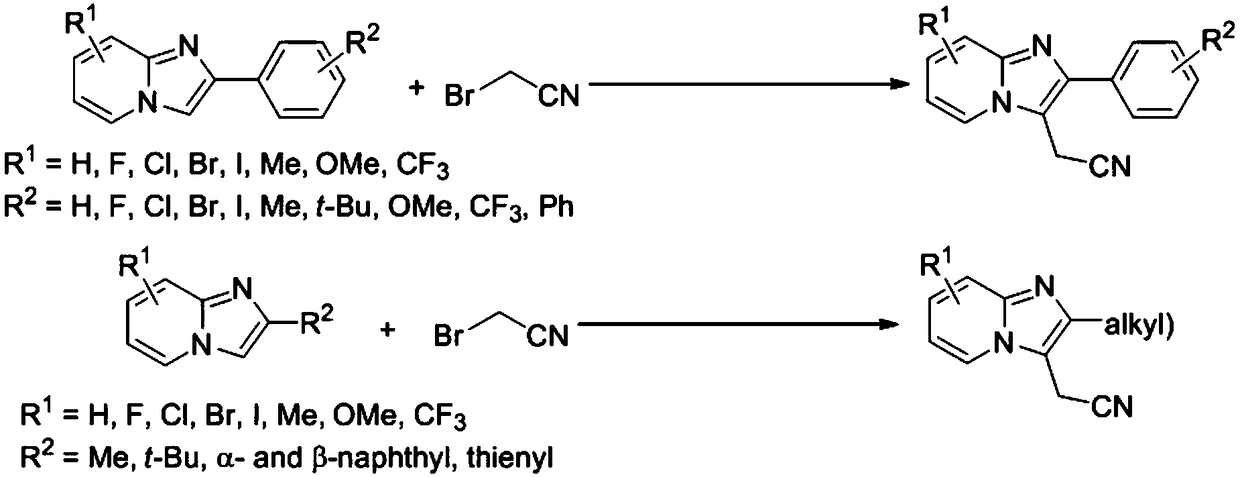
A kind of preparation method of cyanomethylated imidazopyridine compound
The invention discloses a preparation method for a cyanomethylation imidazopyridine compound. The preparation method comprises the steps of using an imidazo[1,2-a]pyridine compound as a raw material, and enabling the imidazo[1,2-a]pyridine compound to react with bromoacetonitrile or iodoacetonitrile under photocatalysis to obtain the cyanomethylation imidazopyridine compound. Compared with the prior art, the method disclosed by the invention avoids using highly toxic products such as potassium cyanide, sodium cyanide or methyl iodide; and in the process of preparing a cyanomethylation product of the imidazo[1,2-a]pyridine compound, the original three steps are cut down to one step in chemical conversion. According to the preparation method disclosed by the invention, the preparation route is short; the preparation method is simple; the production cost is low; the yield of products is high; the use of a solvent and pollution caused to the environment during pollution discharge are reduced; the implementation is easy; and the industrialization is convenient to realize.
Owner:NANJING NORMAL UNIVERSITY
A kind of preparation method of agomelatine intermediate
ActiveCN107353229BReduce turn lossRaw materials are cheap and easy to getCarboxylic acid nitrile preparationOrganic compound preparationOrganic solventGrignard reagent
Owner:XUCHANG HENGSHENG PHARMA
A kind of synthetic method of 6-chloroimidazo[1,2-b]pyridazine-3-carbonitrile
A synthetic method for 6-chloroimidazo[1,2-b]pyridazine-3-carbonitrile, comprising the steps of: Step 1: adding N,N-dimethylformamide dimethyl acetonitrile to the reactor Aldehyde reacts with 3-amino-6-chloropyridazine to obtain N,N-dimethyl-N'-3-(6-chloro-pyridazin) base-formamidine intermediate; step 2: the resulting N,N-dimethyl-N'-3-(6-chloro-pyridazine) base-formamidine intermediate is mixed with solvent, added bromoacetonitrile, reacted, added lye, left standing, precipitated solid, filtered, and obtained Solid mixture; Step 3: The solid mixture obtained in Step 4 is completely dissolved in ethyl acetate, washed with water and saturated brine, dried, filtered, and ethyl acetate is removed to obtain 6-chloroimidazo[1,2-b] Pyridazine‑3‑carbonitrile crude product; step 4: recrystallize 6‑chloroimidazo[1,2‑b]pyridazine‑3‑carbonitrile crude product, filter to obtain 6‑chloroimidazo[1,2‑b ] Pyridazine‑3‑carbonitrile pure product. The whole process of the invention is short in time, and the obtained product has stable quality and high purity.
Owner:HEZE BRANCH QILU UNIV OF TECH(SHANDONG ACAD OF SCI
Method for preparing 3-cyanoethyl-2-hydrocarbyl-4H-benzoxazine from bromoacetonitrile under blue light irradiation condition
The invention discloses a method for preparing 3-cyanoethyl-2-hydrocarbyl-4H-benzoxazine from bromoacetonitrile under a blue light irradiation condition, and belongs to the technical field of alkenylbifunctionalization. The method comprises: adding tris(2-phenylpyridine)iridium, potassium phosphate or potassium carbonate, N-[2-isoalkenylaryl]amide, bromoacetonitrile and a solvent to a treated Schlenk pipe, placing the Schlenk pipe under blue LED light irradiation in a nitrogen atmosphere while stirring for 24 h, terminating the reaction solution with saturated brine, extracting, and carryingout column chromatography to obtain the product. According to the present invention, the 3-cyanoethyl-2-hydrocarbyl-4H-benzoxazine compound is constructed under the blue light LED light irradiation condition by using bromoacetonitrile as the cyanomethyl source, such that the method has advantages of simple and easily available reaction raw materials, simple and easily available catalyst, no requirement of excessive oxidizing agent and equivalent copper salt, mild reaction conditions, high product yield, simple operation, simple post-treatment and the like.
Owner:CHANGZHOU UNIV
A kind of method for preparing gadobutrol
ActiveCN108299322BThe preparation process is simple and gentleSuitable for mass productionOrganic chemistryDodecaneHydrolysate
The invention relates to a method for preparing high-purity Gadobutrol. The method uses 3-(1,4,7,10-tetraazacyclododecane-1-yl)butane-1,2,4-triol to react with bromoacetonitrile or bromoacetamide, anobtained intermediate is hydrolyzed under an alkaline condition, and a hydrolysate is complexed with a gadolinium ion source to obtain the high-purity Gadobutrol. The preparation process of the methodis simple and mild, and the method is suitable for large-scale process production.
Owner:CHEN STONE GUANGZHOU CO LTD
Method for preparing dicyanomethyl phenylphosphine
ActiveCN101974033BHigh purityEasy to produceGroup 5/15 element organic compoundsDichlorophenylphosphineDistillation
The invention discloses a method for preparing dicyanomethyl phenylphosphine, which comprises the following steps of: adding tetrahydrofuran and zinc powder into a reactor, adding dropwise the mixed solution of dichlorophenylphosphine, bromoacetonitrile and the tetrahydrofuran, performing reaction at the constant temperature of 20 to 70 DEG C for 0.5 to 3 hours, performing primary distillation under reduced pressure to recover the tetrahydrofuran and the bromoacetonitrile after the reaction is finished, continuing to add dichloromethane and water into the distilled reactor, performing demixing under stirring to obtain an organic layer, and performing secondary distillation under reduced pressure to remove the dichloromethane to obtain the dicyanomethyl phenylphosphine finished product. The dicyanomethyl phenylphosphine product prepared by the method has the purity of over 98 percent; and the production process is simple, the production is easy to control and the method is favorable for large-scale production.
Owner:SHANDONG UNIV OF SCI & TECH
Preparation method of bromoacetonitrile
PendingCN114751842AAvoid it happening againHigh purityOrganic compound preparationCarboxylic acid nitrile purification/separationBiochemical engineeringReactive distillation
The invention relates to a preparation method of bromoacetonitrile in the technical field of medicine production, which comprises the following steps: reacting cyanomethanesulfonate with brominated salt, and carrying out reactive distillation to obtain a crude product; and rectifying to obtain bromoacetonitrile. According to the present situation that the product quality of the original process is poor, and the crude product contains about 5% of hydroxyacetonitrile, the process of removing water in the reaction system is adopted, so that the generation of hydroxyacetonitrile is avoided, and the rectification efficiency is improved. The novel preparation method provided by the invention overcomes the defects of the existing process, has the advantages of short reaction time, high product purity, high yield and the like, and is suitable for industrial amplification.
Owner:GAOYOU CITY ORGANIC CHEM FACOTRY
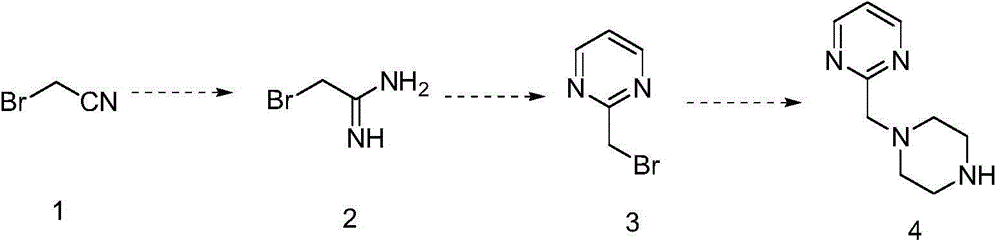
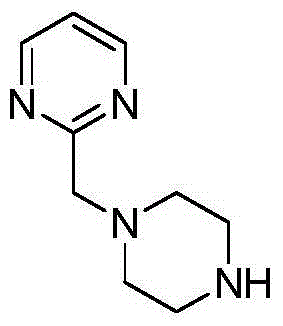
Method for preparing 2- substituted pyrimidine derivative
The invention discloses a method for preparing a 2-substituted pyrimidine derivative 2-(piperazine-1-ylmethyl) pryimidine. The method comprises the following steps: by taking bromoacetonitrile as an initial raw material, performing addition, ring closing and substitution reactions, thereby obtaining a target product 4. The product prepared by using the method can be used as a template micromolecule for synthesizing various compound libraries.
Owner:湖南华腾制药有限公司
Synthesis process of 2-bromo-2, 2-difluoroacetonitrile
PendingCN114014781AAvoid destructionRaw materials are easy to getOrganic compound preparationCarboxylic acid amides preparationEthyl acetateBromoacetonitrile
The invention discloses a synthesis process of 2-bromo-2, 2-difluoroacetonitrile, which comprises the following steps of: 1, reacting ethyl difluorobromoacetate with ammonia gas to generate difluorobromoacetamide; and 2, dehydrating the difluorobromoacetamide under the action of phosphorus pentoxide to generate difluorobromoacetonitrile. The process is safe and reliable, the cost is low, the yield can reach more than or equal to 85%, and the product purity is more than or equal to 99.5%.
Owner:NANTONG BAOKAI CHEM
A method for preparing 3-nitrile ethyl-2-hydrocarbyl-4h-benzoxazine by bromoacetonitrile under blue light irradiation conditions
The invention discloses a method for preparing 3-nitrile ethyl-2-hydrocarbyl-4H-benzoxazine from bromoacetonitrile under the condition of blue light irradiation, and belongs to the technical field of alkenyl difunctionalization. Add tris(2-phenylpyridine)iridium, potassium phosphate or potassium carbonate, N-[2-isoenylaryl]amide, bromoacetonitrile, and solvent to the treated Schlenk tube, and place the Schlenk tube under nitrogen atmosphere. Stir under blue LED light for 24 hours. The reaction solution was terminated by saturated brine, then extracted, and the product was obtained by column chromatography. The present invention uses bromoacetonitrile as the source of nitrile methyl group for the first time, and constructs 3-nitrile ethyl-2-hydrocarbyl-4H-benzoxazine compound under the condition of blue LED light irradiation. The method has the advantages of simple and easy-to-obtain reaction raw materials and catalysts, no need for excessive oxidants and equivalent copper salts, mild reaction conditions, high product yield, simple operation and post-treatment processes, and the like.
Owner:CHANGZHOU UNIV
A kind of preparation method of (e)-oct-4-ene-1,8-dioic acid
ActiveCN109232222BRaw materials are easy to getLow priceCarboxylic acid nitrile preparationOrganic compound preparationChemical synthesisAcid hydrolysis
The invention belongs to the chemical synthesis field, and specifically relates to a preparation method of (E)-oct-4-ene-1,8-diacid. The preparation method comprises the following steps: taking bromoacetonitrile as a starting raw material, reacting with a metal element to obtain a metal composite of bromoacetonitrile, and preparing (E)-oct-4-ene-1,8-dinitrile from the metal composite and 1,4-dibromo-2-butene; and hydrolysing (E)-oct-4-ene-1,8-dinitrile to obtain (E)-oct-4-ene-1,8-diacid. According to the method disclosed by the invention, the starting material is cheap and easily available, and a product with purity of 99% or higher can be obtained by simple post-treatment steps such as liquid-separation extraction and concentration after first-step reaction, and simple extraction after hydrolysing cyano alkali into carboxylic acid or hydrolysing cyan acid into ester in the second step. The process reaction and post-treatment operation are simple, and the obtained product is high in quality, so that using requirements can be met without further purification. The preparation method adopts an environment-friendly process, is economical and practical, and is very suitable for industrial production.
Owner:武汉嘉诺康医药技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthetic method for tert-butyl 1,10-dioxo-2,7-diazaspiro[4.5]decane-7-carboxylate Synthetic method for tert-butyl 1,10-dioxo-2,7-diazaspiro[4.5]decane-7-carboxylate](https://images-eureka.patsnap.com/patent_img/123aef50-11a3-4b9c-87b3-d13af7b7691d/DEST_PATH_IMAGE004.png)
![Synthetic method for tert-butyl 1,10-dioxo-2,7-diazaspiro[4.5]decane-7-carboxylate Synthetic method for tert-butyl 1,10-dioxo-2,7-diazaspiro[4.5]decane-7-carboxylate](https://images-eureka.patsnap.com/patent_img/123aef50-11a3-4b9c-87b3-d13af7b7691d/100002_DEST_PATH_IMAGE002.png)
![8-acetonitrile oxy imidazo [1,2-a] pyridine-3-carbonitrile preparation method 8-acetonitrile oxy imidazo [1,2-a] pyridine-3-carbonitrile preparation method](https://images-eureka.patsnap.com/patent_img/b848e850-d857-4bb6-b505-c0d9597826ca/DEST_PATH_IMAGE001.PNG)


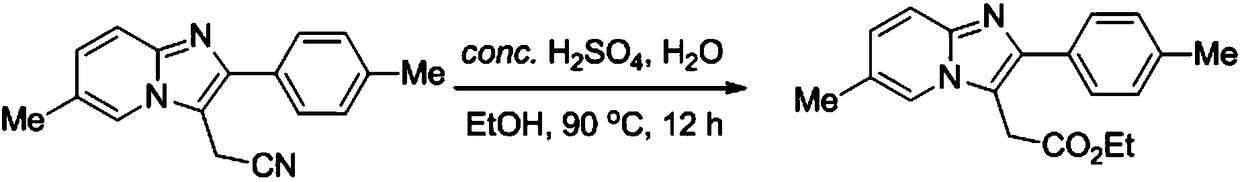
![A kind of synthetic method of 6-chloroimidazo[1,2-b]pyridazine-3-carbonitrile A kind of synthetic method of 6-chloroimidazo[1,2-b]pyridazine-3-carbonitrile](https://images-eureka.patsnap.com/patent_img/34a5b3ee-0b74-4e2b-9529-2f8abae02a4e/HDA0002773882420000011.png)
![A kind of synthetic method of 6-chloroimidazo[1,2-b]pyridazine-3-carbonitrile A kind of synthetic method of 6-chloroimidazo[1,2-b]pyridazine-3-carbonitrile](https://images-eureka.patsnap.com/patent_img/34a5b3ee-0b74-4e2b-9529-2f8abae02a4e/HDA0002773882420000021.png)
![A kind of synthetic method of 6-chloroimidazo[1,2-b]pyridazine-3-carbonitrile A kind of synthetic method of 6-chloroimidazo[1,2-b]pyridazine-3-carbonitrile](https://images-eureka.patsnap.com/patent_img/34a5b3ee-0b74-4e2b-9529-2f8abae02a4e/HDA0002773882420000031.png)