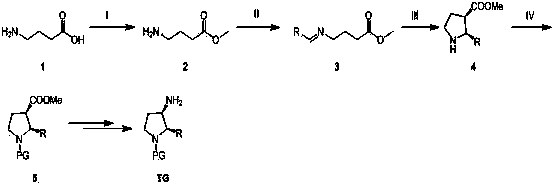
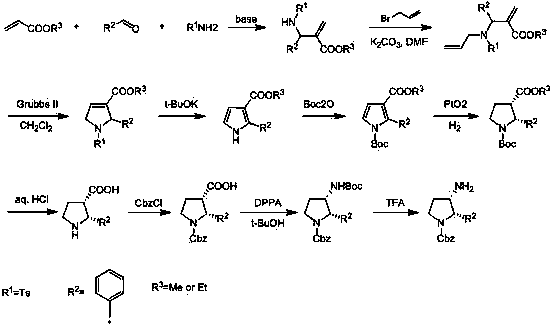
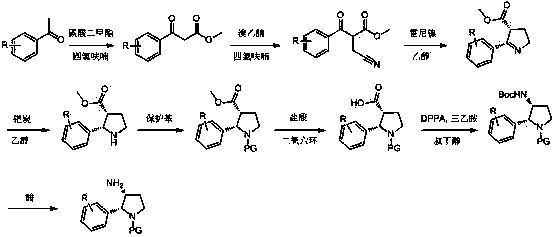
Synthesis method of cis-3-amino-2-arylpyrrolidine derivative
A technology of arylpyrrolidine and synthetic method, which is applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of incapable of large-scale production, harsh reaction conditions, and low yield, and achieve easy industrial operation, fewer reaction steps, and overall The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
[0026] 1. Synthesis of methyl 3-(4-fluorophenyl)-3-oxopropionate
[0027]
[0028] Add anhydrous tetrahydrofuran (540 ml), dimethyl carbonate (235.2 g, 2.61 mol) and sodium hydrogen (130.5 g, 3.263 mol, 60%) into a dry reaction flask, then heat the mixture to reflux, and slowly add A solution of p-fluoroacetophenone (180.3 g, 1.305 mol) in anhydrous tetrahydrofuran (180 ml) was added dropwise within 4 hours. The reaction solution was further refluxed for 0.5 hours. TLC (ethyl acetate:petroleum ether volume ratio=1:6, R f =0.3 ) to monitor the completion of the reaction, the reaction solution was cooled to 0° C., and a mixed solution of 90 ml of methanol and 270 ml of tetrahydrofuran was added dropwise to quench excess sodium hydrogen. Pour the reaction solution into a mixture of saturated ammonium chloride and ice, adjust the pH = 3 with hydrochloric acid, wash with ethyl acetate, water and brine respectively, dry, and concentrate under reduced pressure to ...
Embodiment 2
[0056]
[0057] 1. Synthesis of methyl 2-(cyanomethyl)-3-phenyl-3-oxopropionate
[0058]
[0059] Add anhydrous tetrahydrofuran (200 ml), methyl 3-(4-phenyl)-3-oxopropionate (28.6 g, 0.161 mol) to a dry reaction flask, and carefully add sodium hydrogen (9.6 g , 0.24 mol, 60%) and continued to stir for 0.5 hours. Then bromoacetonitrile (21.2 g, 0.18 mol) and tetrahydrofuran (63 ml) were slowly added dropwise. After the addition was complete, the reaction solution was stirred overnight at room temperature. TLC (ethyl acetate:petroleum ether volume ratio=1:6, R f =0.2 ) to monitor the completion of the reaction. The reaction solution was poured into ice water, adjusted to pH = 3 with hydrochloric acid, extracted with ethyl acetate, washed with water and brine, dried, and concentrated under reduced pressure to obtain a crude product, which was obtained by column chromatography to obtain 2-(cyanomethyl)-3-( Pure methyl 4-phenyl)-3-oxopropionate (24.6 g. 70.4 %).
[006...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com