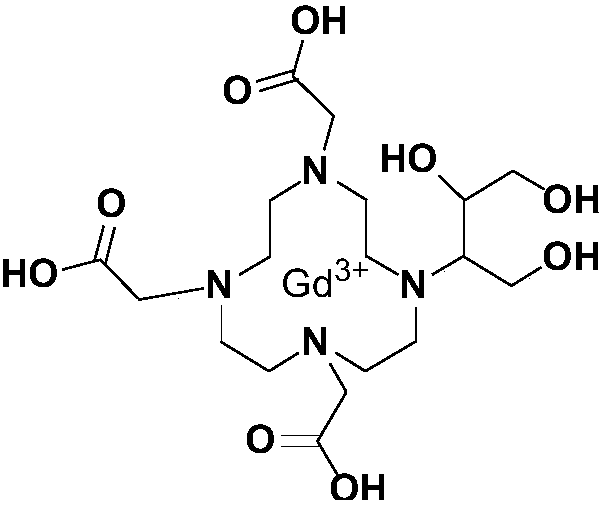
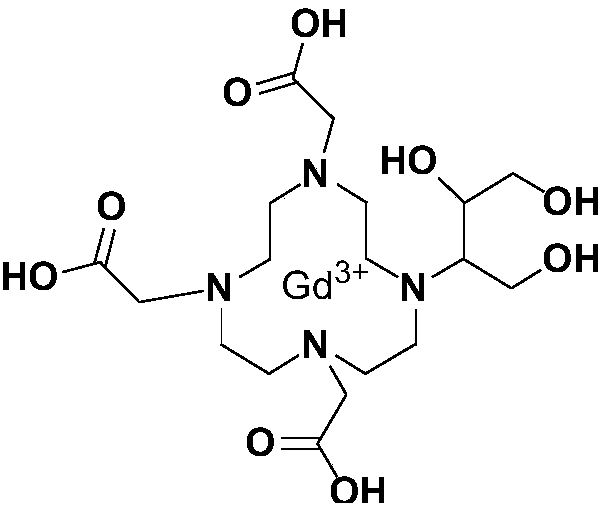
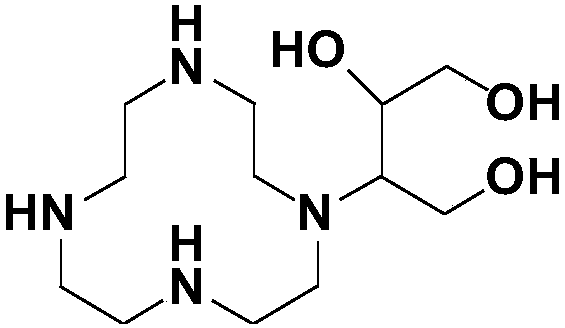
Method for preparing high-purity Gadobutrol
A high-purity technology of gadobutrol, which is applied in the field of preparation of high-purity gadobutrol, can solve problems such as complex methods, and achieve the effects of environmental friendliness, safety risk reduction, and mild and rapid reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0065] Hereinafter, the preparation method of gadobutrol of the present invention will be described in detail.
[0066] According to the preparation method of gadobutrol of the present invention, its synthetic route is as follows:
[0067]
[0068] The preparation of embodiment gadobutrol
[0069] Step 1: 2,2',2"-(10-(1,3,4-Trihydroxybutan-2-yl)-1,4,7,10-tetraazacyclododecane-1,4 ,7-triyl)triacetonitrile
[0070] (1) Use bromoacetonitrile
[0071]
[0072] Add 500mL of acetonitrile into a 1000ml reaction flask, and add 50g (0.1184mol) 3-(1,4,7,10-tetraazacyclododecane-1-yl)butane-1,2 into the reaction flask while stirring , 4-triol 4 hydrochloride, 163g (1.184mol) of potassium carbonate was added to the reaction flask at room temperature, and 42.6g (0.3552mol) of bromoacetonitrile was slowly added thereto. After adding the materials, heat the temperature of the materials in the reaction bottle to 50°C to 55°C, and keep stirring at this temperature for the reaction. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com