Preparation method of agomelatine intermediate
An intermediate, the technology of methoxynaphthalene, which is applied in the field of synthesis of chemical drug intermediates, can solve the problems of high cost, unfavorable to industrialization, medium yield, etc., and achieve the effect of less staff, stable product quality and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
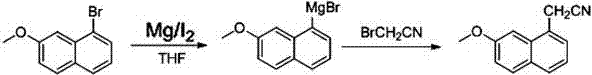
[0019] Put (24.0g, 1.0mol) magnesium chips and 0.1g iodine in a 2L three-necked flask, and add 55ml of tetrahydrofuran and stir evenly, and add 1-bromo-7-methoxynaphthalene (237.1g, 1.0mol ) into 340ml of tetrahydrofuran to make a mixed solution, and slowly add a few drops of the mixed solution to the reaction flask under stirring. After 5 minutes, the solution in the reaction flask boiled slightly, cooled to 5°C in an ice-water bath, and added 340ml of tetrahydrofuran to the reaction flask. Under stirring, start to drop the above-prepared mixed solution, control the reaction temperature to 40° C., and the reaction time to 0.5 h to obtain the reaction solution of 1-bromo-7-methoxy Nagrignard reagent.
[0020] Add (120.0g, 1.0mol) bromoacetonitrile to 340ml tetrahydrofuran to prepare a bromoacetonitrile mixed solution, cool the resulting 1-bromo-7-methoxy Nagrignard reagent reaction solution to 5°C in a reaction flask, and dissolve under stirring The prepared bromoacetonitrile ...
Embodiment 2
[0022] Put (26.4g, 1.1mol) magnesium chips and 0.1g iodine in a 2L three-necked flask, add 60ml tetrahydrofuran and stir evenly, add 1-bromo-7-methoxynaphthalene (237.1g, 1.0mol ) into 360ml of tetrahydrofuran to make a mixed solution, and slowly add a few drops of the mixed solution to the reaction flask under stirring. After 5 minutes, the solution in the reaction flask boiled slightly, cooled to 5°C in an ice-water bath, and added 360ml of tetrahydrofuran to the reaction flask. The mixed solution prepared above was started to be added dropwise under stirring, the reaction temperature was controlled at 38° C., and the reaction time was 2 h to obtain a reaction solution of 1-bromo-7-methoxy Nagrignard reagent.
[0023] Add (132.0g, 1.1mol) bromoacetonitrile to 360ml tetrahydrofuran to prepare a bromoacetonitrile mixed solution, cool the reaction solution of the obtained 1-bromo-7-methoxy Nagrignard reagent to 0°C in a reaction flask, and dissolve under stirring The prepared b...
Embodiment 3
[0025] Put (28.8g, 1.2mol) magnesium chips and 0.1g iodine in a 2L three-necked flask, and add 60ml of dioxane and stir evenly. In the dropping funnel, 1-bromo-7-methoxynaphthalene (237.1g , 1.0mol) into 360ml of dioxane to make a mixed solution, and slowly add a few drops of the mixed solution to the reaction bottle under stirring. After 5 minutes, the solution in the reaction bottle boiled slightly. 360ml of dioxane was added in the middle, and the mixed solution prepared above was added dropwise under stirring, the reaction temperature was controlled at 35°C, and the reaction time was 5h to obtain a reaction solution of 1-bromo-7-methoxy Nagrignard reagent.
[0026] (144.0g, 1.2mol) bromoacetonitrile was added to 360ml of dioxane to prepare a bromoacetonitrile mixed solution, and the resulting 1-bromo-7-methoxy Nagrignard reagent reaction solution was cooled to 5°C in a reaction flask, Slowly drop the bromoacetonitrile mixture into the reaction flask under stirring. After t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com