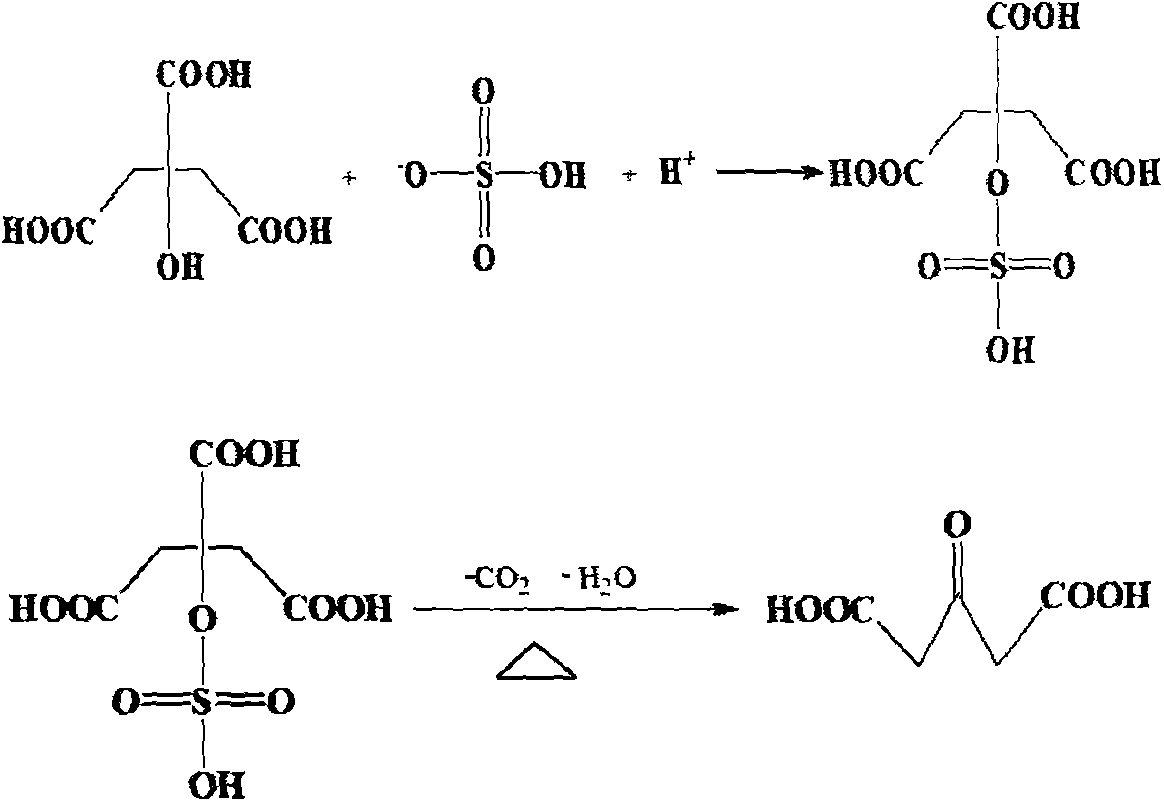
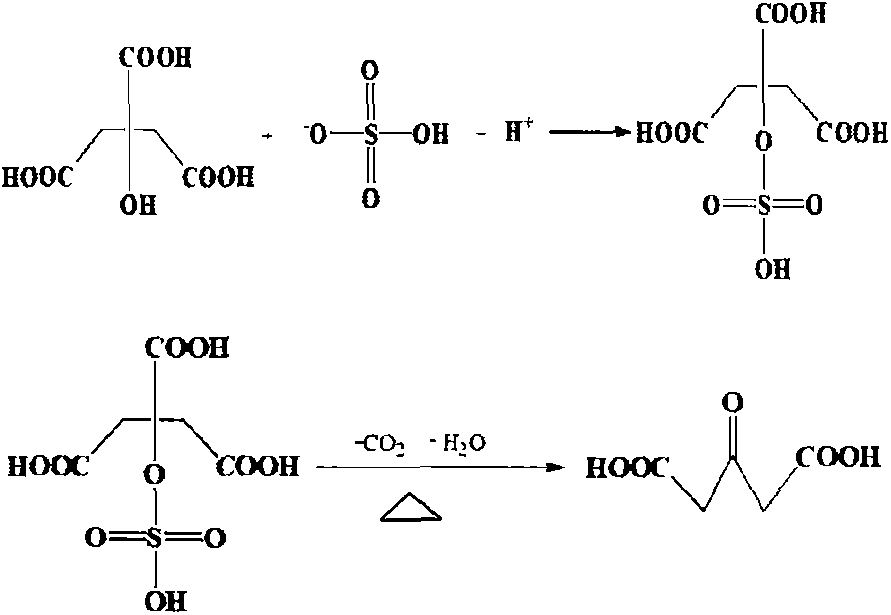
Preparation method for anticholinergic drug intermediate
An intermediate and anticholinergic technology, which is applied in the field of preparation of anticholinergic drug intermediates, can solve the problems of low reaction yield, long reaction time, and low yield, and achieve high yield, stable product quality, High safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 200g of concentrated sulfuric acid into the three-necked flask, stir and cool down to -5°C, take citric acid monohydrate (100.0g, 0.48mol), divide it into three parts, add one part to the concentrated sulfuric acid every 1 hour, and control the reaction at 0~ 30°C, react for 4 hours; take 50.0g of 4A molecular sieve and add to the reaction, continue to react for 4 hours; slowly pour the above reaction solution into 500.0g of ice-water mixture, control the temperature below 50°C , after dropping, cool down to 0°C to crystallize for 6 hours, filter with suction, and dry the filter cake at 60°C to obtain 55.4 g of acetonedicarboxylic acid. Yield 80.2%, purity 99.5%.
Embodiment 2
[0019] Add 500g of concentrated sulfuric acid into the three-necked flask, stir and cool down to -10°C, take citric acid monohydrate (100.0g, 0.48mol), divide it into three parts, add one part to the concentrated sulfuric acid every 1 hour, and control the reaction at 0~ 30°C, react for 8 hours; add 20.0g of phosphorus pentoxide into the reaction, and continue to react for 4 hours; slowly pour the above reaction solution into 800.0g of ice-water mixture, control the temperature at 50 Below 0°C, drop the temperature to 0°C to crystallize for 12 hours, filter with suction, and dry the filter cake at 60°C to obtain 60.9 g of acetonedicarboxylic acid. Yield 88.1%, purity 99.2%.
Embodiment 3
[0021] Add 300g of concentrated sulfuric acid into the three-necked flask, stir and cool down to -5°C, take citric acid monohydrate (100.0g, 0.48mol), divide it into three parts, add one part to the concentrated sulfuric acid every 1 hour, and control the reaction at 0~ 30°C, react for 2 hours; add 50.0g of phosphorus pentoxide into the reaction, and continue to react for 4 hours; slowly pour the above reaction solution into 800.0g of ice-water mixture, control the temperature at 50 Below 0°C, drop the temperature to 0°C to crystallize for 2 hours after dropping, filter with suction, and dry the filter cake at 60°C to obtain 64.0 g of acetonedicarboxylic acid. Yield 92.6%, purity 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com