A kind of preparation method of cyanomethylated imidazopyridine compound
A technology of cyanomethylated imidazole and methylated imidazole, which is applied in the field of medicinal chemistry, can solve the problems of low atom utilization rate, industrial production limitation, and long reaction steps, and achieve the effects of easy implementation, less pollution, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
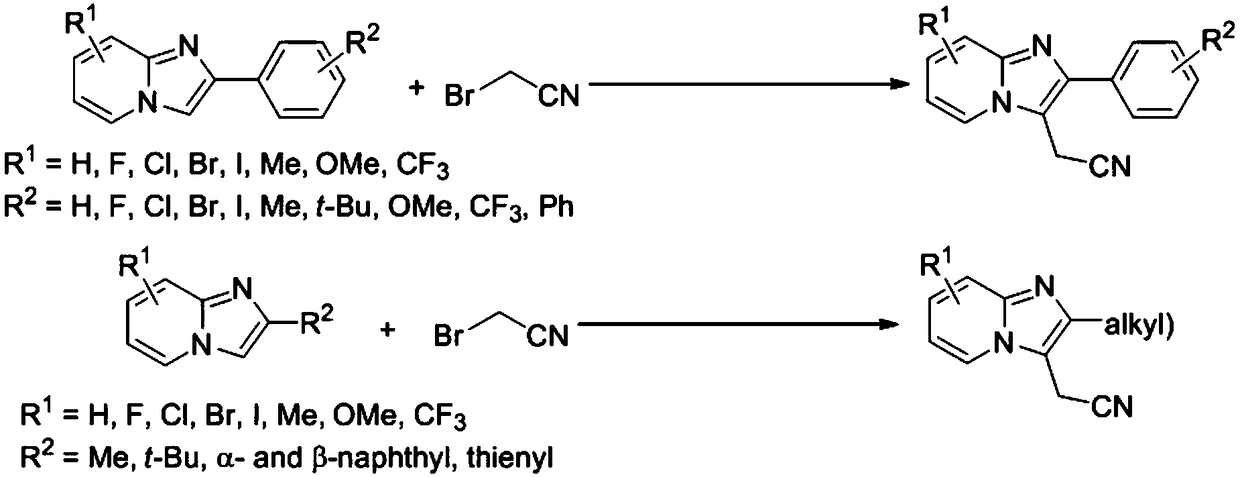
[0025] Example 1 Preparation of 3-cyanomethyl-2-phenylimidazo[1,2-a]pyridine
[0026] Step 1. Under nitrogen protection, add 2-phenylimidazo[1,2-a]pyridine (0.97g, 5mmol) into a 100mL reaction flask, add fac-Ir(ppy) 3 (65.5mg, 2mol%), sodium bicarbonate (0.84g, 10mmol), bromoacetonitrile 1.39mL (2.40g, 20mmol), 30mL dimethyl sulfoxide as a solvent, react at room temperature, under the irradiation of a 5W blue LED lamp, Stir for 12 hours, add water to quench the reaction, extract with dichloromethane (60mL×3), wash with 50mL saturated brine, dry over anhydrous sodium sulfate, concentrate under vacuum, purify and isolate white solid 3-cyanoform Base-2-phenylimidazo[1,2-a]pyridine 1.01g, the yield is 87%, the structural detection data of this compound are as follows:
[0027] m.p.100-101°C (reported m.p.111°C); 1 H NMR (400MHz, CDCl3): δ (ppm) 8.05 (d, J = 8.8Hz, 1H), 7.72–7.67 (m, 3H), 7.53–7.48 (m, 2H), 7.45–7.40 (m, 1H) ,7.34–7.30(m,1H),7.00(td,J=6.8,1.2Hz,1H),4.15(s,2H).13...
Embodiment 2
[0028] Example 2 Preparation of 3-cyanomethyl-2-(4-methylphenyl)-imidazo[1,2-a]pyridine
[0029]As in Example 1, under nitrogen protection conditions, 2-(4-methylphenyl)-imidazo[1,2-a]pyridine (1.04g, 5mmol) was added to a 100mL reaction flask, and fac-Ir( ppy) 3 (65.5mg, 2mol%), sodium bicarbonate (0.84g, 10mmol), bromoacetonitrile 1.39mL (2.40g, 20mmol), 30mL dimethyl sulfoxide as a solvent, react at room temperature, under the irradiation of a 5W blue LED lamp, Stir for 12 hours, add water to quench the reaction, extract with dichloromethane (60mL×3), wash with 50mL saturated brine, dry over anhydrous sodium sulfate, concentrate under vacuum, purify and isolate white solid 3-cyanoform Base-2-(4-methylphenyl)-imidazo[1,2-a]pyridine 1.19g, the yield is 96%, the structural detection data of this compound are as follows:
[0030] m.p.123-124°C; 1 H NMR (400MHz, CDCl3): δ (ppm) 8.08 (d, J = 6.8Hz, 1H), 7.75 (d, J = 9.0Hz, 1H), 7.61 (d, J = 8.0Hz, 2H), 7.37– 7.33(m,3H),7.02(t...
Embodiment 3
[0031] Example 3 Preparation of 3-cyanomethyl-2-(4-methoxyphenyl)-imidazo[1,2-a]pyridine
[0032] As in Example 1, under nitrogen protection conditions, 2-(4-methoxyphenyl)-imidazo[1,2-a]pyridine (1.12g, 5mmol) was added to a 100mL reaction flask, and fac-Ir (ppy) 3 (65.5mg, 2mol%), sodium bicarbonate (0.84g, 10mmol), bromoacetonitrile 1.39mL (2.40g, 20mmol), 30mL dimethyl sulfoxide as a solvent, react at room temperature, under the irradiation of a 5W blue LED lamp, Stir for 12 hours, add water to quench the reaction, extract with dichloromethane (60mL×3), wash with 50mL saturated brine, dry over anhydrous sodium sulfate, concentrate under vacuum, purify and isolate white solid 3-cyanoform Base-2-(4-methoxyphenyl)-imidazo[1,2-a]pyridine 1.25g, the yield is 95%, the structural detection data of this compound are as follows:
[0033] mp 120–121°C; 1 H NMR (400MHz, CDCl 3 ): δ(ppm)8.06(d,J=6.8Hz,1H),7.73(d,J=9.1Hz,1H),7.65(d,J=8.6Hz,2H),7.37–7.32(m,1H) ,7.07–6.99(m,3H),4.16...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com