New synthetic method of pesticide fludioxonil intermediate
A technology for fludioxonil and intermediates, which is applied in the new synthesis field of pesticide fludioxonil intermediates, can solve the problems of serious pollution of three wastes, poor comprehensive benefits, long cycle, etc., and achieve the effect of simple operation, convenient treatment and simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
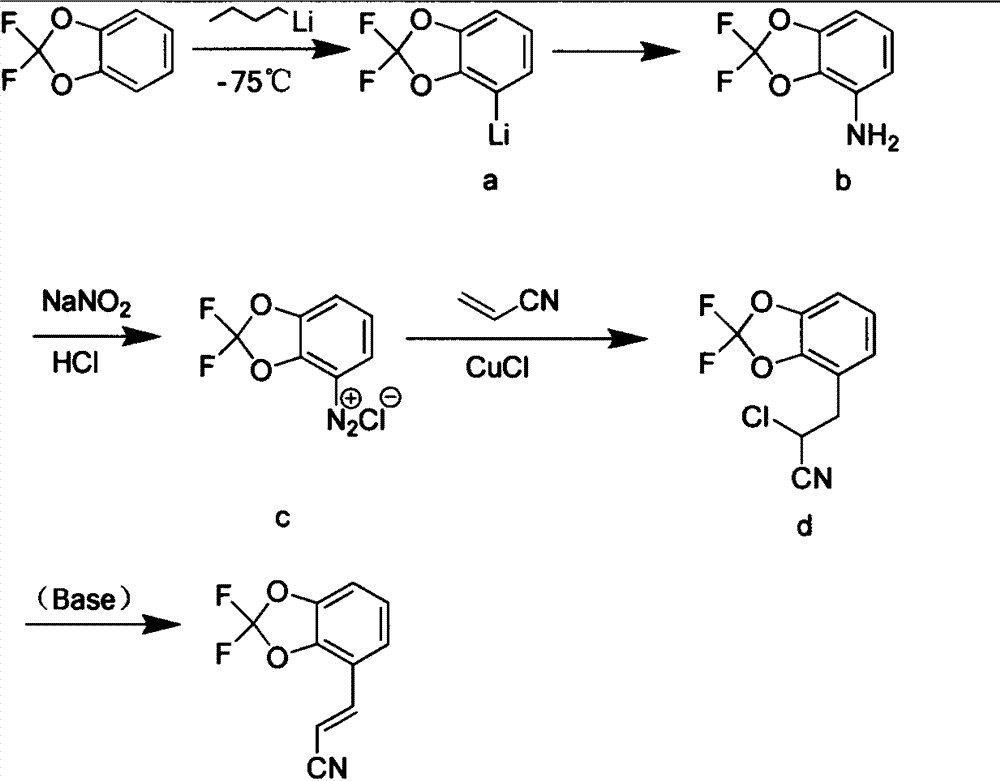
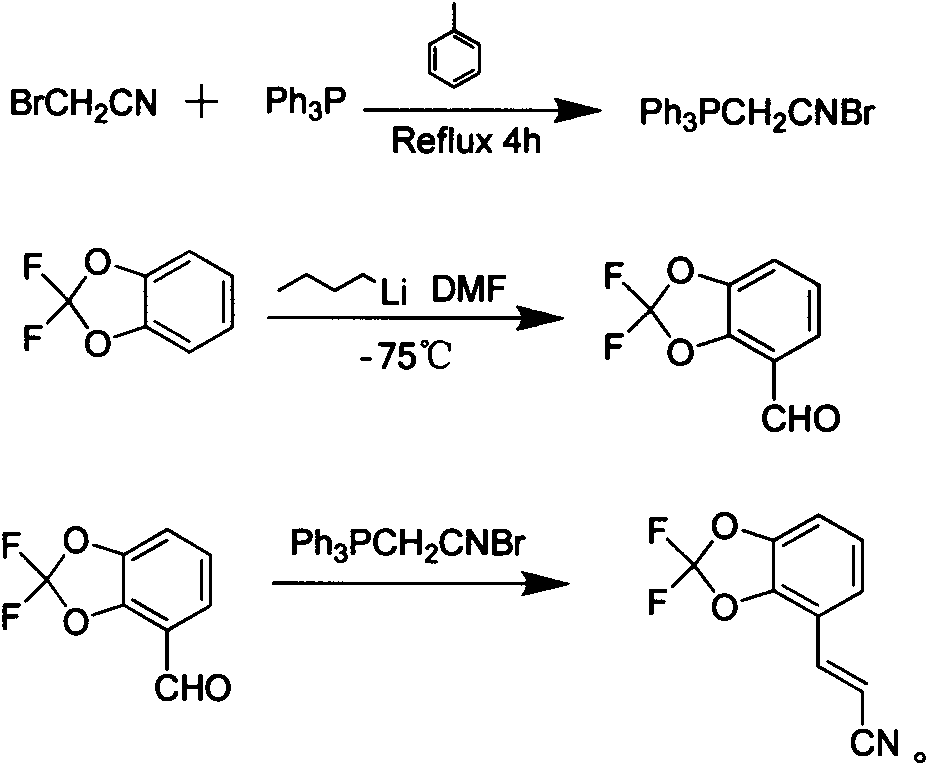
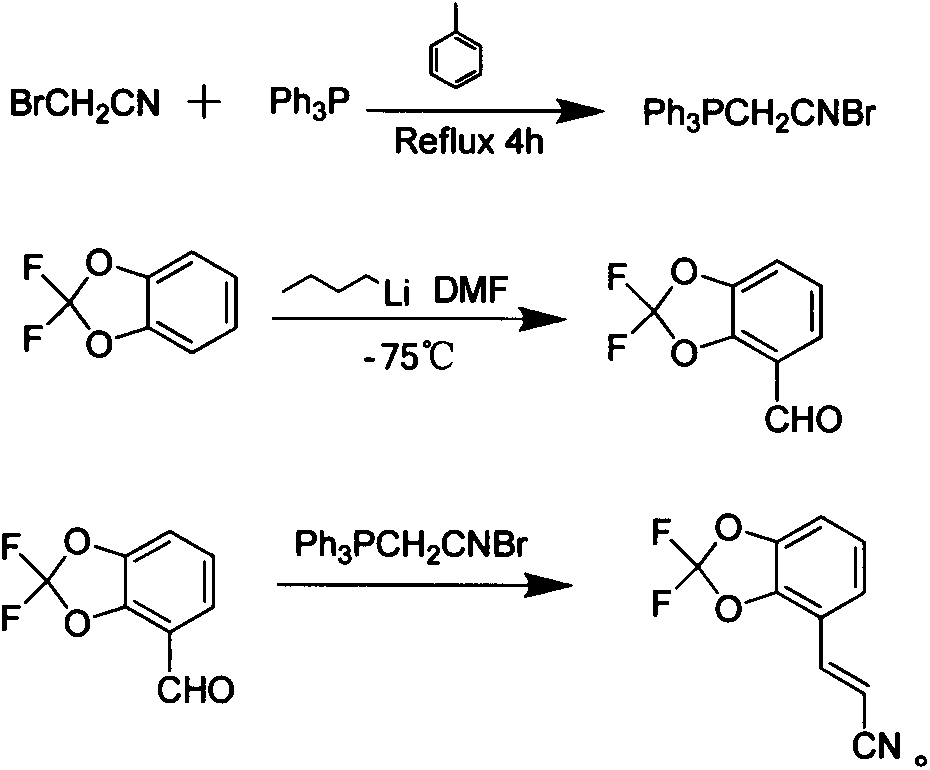
[0023] The new synthetic method of pesticide fludioxonil intermediate comprises the following steps:
[0024] Step 1) adding triphenylphosphine to the toluene solution of bromoacetonitrile, and after reflux reaction for 4 hours, filter to obtain bromoacetonitrile triphenylphosphine salt white solid;
[0025] Step 2) At low temperature, slowly dropwise add a cyclohexane solution of n-butyllithium into the tetrahydrofuran solution of 2,2-difluoro-1,3-benzodioxol, react for 1 hour, then slowly add N , N-dimethylformamide, then reacted for 30 minutes, then slowly warmed up to room temperature, after 30 minutes, quenched the reaction with 2mol / L hydrochloric acid solution, extracted with ethyl acetate, washed the organic layer with saturated saline, and anhydrous sulfuric acid Drying over sodium and evaporating the solvent gave 4-formyl-2,2-difluoro-1,3-benzodioxol;
[0026] Step 3) Dissolve 4-formyl-2,2-difluoro-1,3-benzodioxol and bromoacetonitrile triphenylphosphonium salt in d...
Embodiment 1
[0030] Step 1) 10 grams of bromoacetonitrile was added to 300 milliliters of toluene, then 21.8 grams of triphenylphosphine was added, and after reflux reaction for 4 hours, the white precipitate was filtered to obtain 30.5 grams of bromoacetonitrile triphenylphosphonium salt;
[0031] Step 2) 15.8 grams of 2,2-difluoro-1,3-benzodioxol was added to 200ml of dry tetrahydrofuran, cooled to -75°C under nitrogen protection, and then slowly added dropwise into 6.4 grams of n-butyllithium After reacting for 1 hour, slowly add 7.31 g of N,N-dimethylformamide dropwise, react for 30 minutes after the drop, then slowly warm up to room temperature, after 30 minutes, use 2mol / L hydrochloric acid The reaction was quenched by the solution, extracted with ethyl acetate, the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was evaporated to obtain 16.5 g of 4-formyl-2,2-difluoro-1,3-benzodioxol;
[0032] Step 3) Dissolve 10 grams of 4-formyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com