Patents
Literature
81results about How to "Reasonable reaction process design" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate
ActiveCN111620869AReasonable reaction process designMethod route shortOrganic chemistryBulk chemical productionPalladium on carbonNonane
The invention relates to a synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate, and mainly solves the technical problem that no suitable industrial synthesis method exists at present. The method comprises the following seven steps: 1, reacting a compound 1 with ethyl malonate added into a solvent ethanol to obtain a compound 2; 2, reacting the compound 2 with lithium borohydridein tetrahydrofuran to obtain a compound 3; 3, reacting the compound 3 with p-toluenesulfonyl chloride in dichloromethane to obtain a compound 4; 4, adding cesium carbonate into the compound 4 in acetonitrile serving as a solvent for cyclization to obtain a compound 5; 5, adding magnesium chips into the compound 5 in methanol serving as a solvent for reduction to obtain a compound 6, 6, reacting the compound 6 with Boc anhydride in dichloromethane to obtain a compound 7, and 7, reacting the compound 7 with palladium on carbon in methanol to obtain a final compound 8.
Owner:SHANGHAI STA PHARMA R&D CO LTD +1
Synthesis method of 8-oxo-5-oxa-2-diazaspiro [3.5] nonane-2-tert-butyl formate
InactiveCN107383038AReasonable reaction process designShort routeOrganic chemistryChemistryTert-Butyl formate
The invention relates to a synthesis method of 8-oxo-5-oxa-2-diazaspiro [3.5] nonane-2-tert-butyl formate, and mainly solves the technical problem that no industrial method suitable for industrial synthesis exists in the prior art. The synthesis method comprises three steps that 1, a compound 1, a compound 2 and TMEDA react in a solvent of tetrahydrofuran to obtain a compound 3; 2, the compound 3 is subjected to intramolecularly ring closing to obtain a compound 4 under the gathering effects of chlorine tosylate by using n-butyllithium as the alkali; 3, the compound 4 and ozone react to obtain a final compound 5. A reaction formula is shown in the specification.
Owner:SHANGHAI STA PHARMA R&D CO LTD
Synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate
InactiveCN109503605AReasonable reaction process designThe method route is simpleOrganic chemistrySynthesis methodsEthyl acetate
The invention relates to a synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate. The technical problem that an appropriate industrial synthesis method is inexistent at presentis mainly solved. The synthesis method is divided into two steps: the first step, adding triethylamine in a methanol solution dissolved with a compound 1 and amino ethyl acetate hydrochloride, and then dropwise adding tetraisopropyl titanate in the reaction solution, after completing the dropwise adding, reacting the mixed system for 12 h at 25 DEG C; adding trimethylsilyl cyanide in the reactionsystem at 25 DEG C, and then reacting for 12 h at 25 DEG C, performing post-treatment to obtain a compound 2; and the second step, adding the compound 2, nickel and ammonia water in the methanol solution, and performing hydrogenation to obtain the final compound.
Owner:上海合全医药有限公司
Synthesis method of 7-hydroxymethyl-2,5-diazaspiro [3,4] octane-2-dimethylethylester
ActiveCN107383026AReasonable reaction process designEasy to getOrganic chemistryTriethylphosphiteSynthesis methods
The invention relates to a synthesis method of a compound of 7-hydroxymethyl-2,5-diazaspiro [3,4] octane-2-dimethylethylester, and mainly solves the technical problem that no industrial method suitable for industrial synthesis exists in the prior art. A compound 1 and triethyl-phosphite are used as raw materials for synthesizing and obtaining the final compound through eight steps. A reaction formula is shown in the specification.
Owner:成都药明康德新药开发有限公司
Synthetic method for tert-butyl 1,10-dioxo-2,7-diazaspiro[4.5]decane-7-carboxylate
InactiveCN109608460AReasonable reaction process designHigh yieldOrganic chemistryCarboxylic saltCatalytic effect
The invention relates to a synthetic method for tert-butyl 1,10-dioxo-2,7-diazaspiro[4.5]decane-7-carboxylate, and mainly solves the technical problem that the tert-butyl 1,10-dioxo-2,7-diazaspiro[4.5]decane-7-carboxylate has no synthetic method suitable for industrialization. The synthetic method comprises the following four steps: step 1, dissolving a compound 1 and bromoacetonitrile into anhydrous acetone, and adding potassium carbonate into the above reaction system to obtain a compound 2; step 2, performing a reaction on the compound 2 under the action of sodium borohydride to obtain a compound 3; step 3, introducing hydrogen, and performing ring closing on the compound 3 under the catalytic effect of nickel to obtain a compound 4; and step 4, performing oxidation on the compound 4 toobtain a compound 5, wherein a reaction formula is shown in the description.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
6-(tert-butoxycarbonyl) octahydro-furo [2,3-c] pyridine-4-carboxylic acid synthetic method
ActiveCN105669686AReasonable reaction process designShort synthetic routeOrganic chemistryFuranTert-Butyloxycarbonyl protecting group
The present invention relates to a 6-(tert-butoxycarbonyl) octahydro-furo [2,3-c] pyridine-4-carboxylic acid synthetic method and mainly solves a technical problem that currently no suitable industrial synthetic method exists. The synthetic method comprises ten steps as follows: firstly a compound 1 is subjected to a reductive amination with a furfural to obtain a compound 2; then the compound 2 is subjected to alkylation to produce a compound 3; the compound 3 is hydrolyzed to obtain a hydrolyzate 4; the hydrolyzate 4 is subjected to an intramolecular Friedel-Crafts reaction to obtain a compound 5; the compound 5 is subjected to a hydrogenation reduction to obtain a compound 6; a trifluoromethanesulfonyl is bond to a ketone group of the compound 6 to obtain a compound 7; a carbonyl group inserted to the compound 7 to obtain a compound 8; the compound 8 is subjected to a double bond hydrogenation reduction to obtain a compound 9; the compound 9 is subjected to ethoxycarbonyl removing by a strong acid; and the ethoxycarbonyl removed compound 9 is reacted with a Boc anhydride to obtain a final compound 11. The reaction formulas are described as follows.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
Synthesis method of cis5-tert-butyl-3A-methyl-tetrahydro 1H-furan[3,4 c] pyrrole 3A,5(3H) dicarboxylic ester
InactiveCN107383033AReasonable reaction process designMethod route shortOrganic chemistry methodsFuranDiisopropyl azodicarboxylate
The invention relates to a synthesis method of cis5-tert-butyl-3A-methyl-tetrahydro 1H-furan[3,4 c] pyrrole 3A,5(3H) dicarboxylic ester. The method mainly solves the technical problem that no proper industrial synthesis method exists in the prior art. The method comprises five steps that: 1, a compound 1 uses sodium borohydride as a reducing agent to react in an ethanol solvent to obtain a compound 2; 2, the compound 2 uses tetrahydrofuran as a solvent to obtain a compound 3 under the effects of triphenylphosphine and diisopropyl azodicarboxylate; 3, the compound 3 and the N-methoxymethyl-N-(trimethylsilyl) benzylamine use dichloromethane as a solvent to obtain a compound 4 through room temperature reduction under the action of trifluoroacetic acid; 4, the compound 4 and thionyl chloride react under the methanol backflow condition to obtain a compound 5; 5, the compound 5 uses palladium hydroxide catalysts and Boc anhydride auxiliary agents to obtain a final compound 6 through catalytic hydrogenation reaction.
Owner:上海药明康德新药开发有限公司 +4
Synthesis method of 8-(tert-butyloxycarbonyl)-6, 7, 8, 9-tetrahydro-5-hydro-imidazole[1, 5-a][1, 4]diaza-6-carboxylic acid
ActiveCN105693727AReasonable designThe reaction conditions are stable and easy to controlOrganic chemistryCarbonyl groupOrganic synthesis
The invention relates to a synthesis method of 8-(tert-butyloxycarbonyl)-6, 7, 8, 9-tetrahydro-5-hydro-imidazole[1, 5-a][1, 4]diaza-6-carboxylic acid. In the method, 8-(tert-butyloxycarbonyl)-6, 7, 8, 9-tetrahydro-5-hydro-imidazole[1, 5-a][1, 4]diaza-6-carboxylic acid is synthesized from easily-available 1-hydroimidazole-4, 5-dicarboxylic acid as the raw material by ten steps. And 8-(tert-butyloxycarbonyl)-6, 7, 8, 9-tetrahydro-5-hydro-imidazole[1, 5-a][1, 4]diaza-6-carboxylic acid is a useful organic synthesis intermediate.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD +2
Synthetic method of 7-tert-butyl 3-ethyl 8-methyl-5,6 dihydrotriazolopyrazine 3,7(8H) phthalate ester
InactiveCN108033966AReasonable reaction process designLower synthesis costOrganic chemistryEthyl phosphateMethyl group
The invention relates to a preparation method of 7-tert-butyl 3-ethyl 8-methyl-5,6 dihydrotriazolopyrazine 3,7(8H) phthalate ester and mainly solves the technical problems that at present, a compoundis not suitable for an industrial preparation method. The preparation method comprises the following steps: taking 2-chloro-3-methylpyrazine as a starting raw material and preparing the 7-tert-butyl-3-ethyl-8-methyl-5,6-dihydro-[1,2,4]triazol[4,3-a]pyrazine-3,7(8H)-phthalate ester through three-step reaction. A reaction formula is shown in the Specification. The 7-tert-butyl-3-ethyl-8-methyl-5,6-dihydro-[1,2,4]triazol[4,3-a]pyrazine-3,7(8H)-phthalate ester obtained by the preparation method provided by the invention is a useful intermediate or a product for synthesis of multiple drugs.
Owner:WUXI APPTEC (TIANJIN) CO LTD
Synthesis method of tert butyl-1-(hydroxymethyl)-3-oxa-9-azaspiro-[5.5] undecane-9-formyl ester
ActiveCN107216335AReasonable reaction process designLower synthesis costOrganic chemistrySynthesis methodsCombinatorial chemistry
The present invention relates to a synthesis method of tert butyl-1-(hydroxymethyl)-3-oxa-9-azaspiro-[5.5] undecane-9-formyl ester, and mainly solves the technical problems of low imperfect rate, no easiness in control of reaction, experimental operation inconvenience and the like of the synthesis process in the prior art. The tert butyl-1-(hydroxymethyl)-3-oxa-9-azaspiro-[5.5] undecane-9-formyl ester is prepared from 1-phenylmethyl piperidine-4-one as a starting raw material by a three-step reaction. The reaction formula is as shown in the specification, and the product obtained by the method is a useful intermediate or product for synthesis of many pharmaceuticals.
Owner:上海药明康德新药开发有限公司 +4
Synthesis method of spiro[2.5]octane-5-carboxylic acid
InactiveCN103102261AReasonable reaction process designLower synthesis costPreparation from nitrilesCyclohexenoneGrignard reagent
The invention relates to a synthesis method of spiro[2.5]octane-5-carboxylic acid, and is used for solving the technical problems of the existing method that the synthesis operation is difficult and the raw materials are expensive. A conventional industrial raw material 1,3-cyclohexanedione is used as a start raw material which reacts with methanol through the catalysis of concentrated sulfuric acid to obtain 3-methoxy-cyclohexenone; 3-methoxy-cyclohexenone is cyclized with an ethyl Grignard reagent in the presence of Lewis acid to obtain 5-methoxyspiro[2.5]oct-4-ene; in the presence of p-toluenesulfonic acid, 5-methoxyspiro[2.5]oct-4-ene is stirred in tert-methyl ether at room temperature to obtain spiro[2.5]oct-5-one; and in the presence of alkali and ethanol, spiro[2.5]oct-5-one reacts with p-tosyl isonitrile at 50 DEG C by taking dimethoxyethane as a solvent to obtain spirao[2.5]oct-5-nitrile; and reflux hydrolysis is performed in an alkaline condition to obtain spiro[2.5]octane-5-carboxylic acid.
Owner:上海药明康德新药开发有限公司 +2
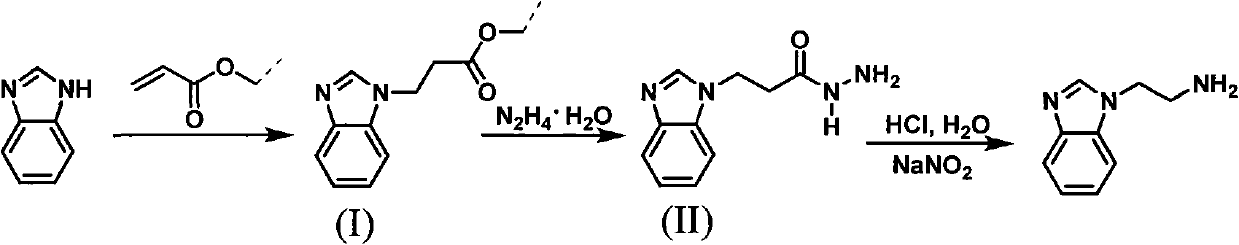
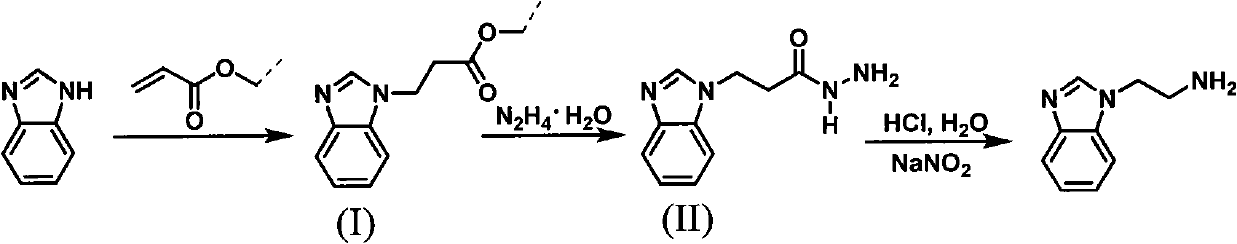
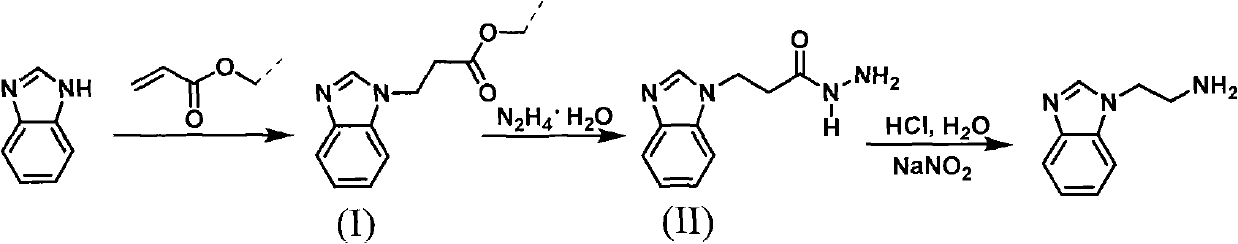
Method for synthesizing 2-(1-benzimidazolyl) ethylamine
InactiveCN101973942AReasonable reaction process designSmooth responseOrganic chemistryEtherRearrangement reaction
The invention discloses a method for synthesizing 2-(1-benzimidazolyl) ethylamine, comprising the following steps of: with a conventional industrial raw material of benzimidazole as an initial raw material, firstly, subjecting the benzimidazole and acrylic resin to a Michael addition reaction to prepare an intermediate of (I) 2-(1-benzimidazolyl) propionic ether; secondly, subjecting the intermediate (I) to a hydrazinolysis reaction to prepare an intermediate (II) 2-(1-benzimidazolyl) propionamide; and finally, subjecting the intermediate (II) to a Kertesz rearrangement reaction to obtain a target object of 2-(1-benzimidazolyl) ethylamine. The synthetic path is shown In the specification. The invention has the advantages of stable reaction, mild conditions and high total yield and is convenient to operate and control.
Owner:EAST CHINA NORMAL UNIV
Preparation method of 4-methyl formate-2-oxo-1,8-diazaspiro[4.5]decane-8-tert-Butyl formate
InactiveCN109651368AReasonable reaction process designLower synthesis costOrganic chemistryMonoethyl maleatePotassium fluoride
The invention relates to a preparation method of 4-methyl formate-2-oxo-1,8-diazaspiro[4.5]decane-8-tert-Butyl formate and mainly solves the technical problem that there is no method suitable for industrial synthesis so far. The preparation method comprises the following two steps: Step 1, a compound 1 and dimethyl maleate react under the action of tetrabutylammonium iodide and potassium fluoridewith dimethyl sulfoxide used as a solvent to generate a compound 2; and Step 2, the compound 2 is subjected to raney nickel / H2 catalytic hydrogenation in methanol used as a solvent to obtain a compound 3, namely 4-methyl formate-2-oxo-1,8-diazaspiro[4.5]decane-8-tert-Butyl formate. The reaction equation is as shown in the specification.
Owner:武汉药明康德新药开发有限公司
Synthetic method of 6-oxo-2-azaspiro[3,3]heptane-2-t-butyl carboxylate
InactiveCN102442934AHas the value of large-scale preparationReasonable reaction process designOrganic chemistryChemical synthesisKetone
The invention relates to the chemical synthesis field, and concretely relates to a synthetic method of 6-oxo-2-azaspiro[3,3]heptane-2-t-butyl carboxylate. The synthetic method is characterized in that the synthetic method comprises the following steps: 1, reducing a raw material compound (14) with lithium aluminum hydride; 2, selecting Ts (toluene sulfonyl) protection; 3, carrying out ring closure on the substance obtained in step 2 and o-nitrobenzenesulfonamide under the action of potash to obtain a compound (19); 4, reacting the compound (19) with thiophenol in a solvent DMF (dimethylformamide) under the action of potash to obtain a compound (20); and 5, generating a ketone under acidic conditions, and reacting the ketone with BOC2O (di-tert-butyl dicarbonate) under alkaline conditions to obtain 6-oxo-2-azaspiro[3,3]heptane-2-t-butyl carboxylate. The preparation method of the invention, which allows the yield to be high, the whole yield to reach 41% and conditions to be mild, is a synthetic method with large scale preparation values.
Owner:PHARMABLOCK SCIENCES (NANJING) INC
Method for preparing 5-(tert-butoxycarbonyl)-2-oxooctahydropyrrolo[3, 4-b]pyrrole-3a-carboxylic acid
InactiveCN109503593AReasonable reaction process designThe method route is simpleOrganic chemistry methodsSodium bicarbonateTert-Butyloxycarbonyl protecting group
The invention relates to a method for synthesizing (3aS, 6aR)-5-(tert-butoxycarbonyl)-2-oxooctahydropyrrolo[3, 4-b]pyrrole-3a-carboxylic acid, and mainly solves a technical problem of absence of a method suitable for industrial synthesis at present. The method provided by the invention comprises the following four steps: step one, dissolving a compound 1, ethyl bromoacetate and tetrabutylammoniumfluoride in tetrahydrofuran to obtain a compound 2; step two, dissolving the compound 2, hydroxylamine hydrochloride and sodium bicarbonate in a mixed solvent of tetrahydrofuran and ethanol to obtaina compound 3; step three, adding the compound 3, Raney nickel and ammonia water into ethanol, filtering and spin-drying to obtain a compound 4 after finishing reaction; and step four, adding the compound 4 and sodium ethoxide into ethanol, performing reflux reaction to obtain a white solid compound 5, namely, (3aS, 6aR)-5-(tert-butoxycarbonyl)-2-oxooctahydropyrrolo[3, 4-b]pyrrole-3a-carboxylic acid.
Owner:武汉药明康德新药开发有限公司
Synthetic method for 1-(ethoxycarbonyl)imidazo[1,5]pyridine-6-carboxylic acid
InactiveCN110183442AReasonable reaction process designMethod route shortOrganic chemistryN dimethylformamideCarboxylic acid
The invention relates to a synthetic method for 1-(ethoxycarbonyl)imidazo[1,5]pyridine-6-carboxylic acid, and mainly solves the technical problem that a current method is not suitable for industrial synthesis. The method comprises the following three steps: step 1, firstly performing a reaction on a compound 1 and di-tert-butyl dicarbonate in solvent tetrahydrofuran under the action of 4-dimethylaminopyridine to obtain a compound 2; step2, performing a reaction on the compound 2 and ethyl cyanoacetate in solvent N,N-dimethylformamide under the action of cesium carbonate to obtain a compound 3;and step 3, performing a reaction on the compound 3 under the action of hydrochloric acid-ethyl acetate to obtain the 1-(ethoxycarbonyl)imidazo[1,5]pyridine-6-carboxylic acid. The reaction formula isshown in the description.
Owner:WUXI APPTEC
Synthesis method of 1,7-diazaspiro[3.5]nonane-7-tert-butyl formate
InactiveCN113214256AReasonable reaction process designShort synthetic routeOrganic chemistryBulk chemical productionSulfonyl chlorideNonane
The invention provides a process synthesis method of 1,7-diazaspiro[3.5]nonane-7-tert-butyl formate. The synthesis method comprises the following five steps: 1, reacting a compound 1 with a bromine compound 2 and ammonium acetate by a one-pot method to obtain a compound 2; 2, reducing a compound 3 by using lithium borohydride to obtain a compound 4; 3, using triethylamine is used as alkali, and reacting the compound 4 with p-toluene sulfonyl chloride to generate a compound 5; 4, under the catalysis of potassium iodide, taking cesium carbonate as alkali, and carrying out ring closing on the compound 5 to generate a compound 6; and 5, under the action of magnesium chips, removing p-toluene sulfonyl from the compound 6 to obtain a compound 7. The method mainly solves the technical problem that no method suitable for industrial synthesis exists at present, and has the advantages that raw materials are easy to obtain, the total yield is high, and the method is suitable for industrial production.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
Preparation method of tert-butyl-1-oxo-6-oxa-9-azaspiro[4.5]decane-9-carboxylate
InactiveCN109608411AReasonable reaction process designThe method route is simpleOrganic chemistrySynthesis methodsCarboxylic salt
The invention relates to a preparation method of tert-butyl-1-oxo-6-oxa-9-azaspiro[4.5]decane-9-carboxylate, and mainly solves the technical problem that no suitable industrial synthesis method existsat present. The preparation method comprises the following five steps: step 1, dissolving a compound 1 in tetrahydrofuran, dropwise adding lithium hexamethyldisilazide under protection of nitrogen, then adding bromopentene, and after the reaction is finished, treating the obtained crude product compound 2; step 2, adding potassium permanganate into a mixed solvent of the compound 2, sodium periodate and acetone in batches, and carrying out treatment after a reaction is completed to obtain a compound 3; step 3, dissolving the compound 3 and cesium carbonate in acetonitrile, dropwise adding iodomethane, and carrying out treatment after a reaction is completed to obtain a compound 4; step 4, sequentially adding the compound 4 and potassium tert-butoxide into tetrahydrofuran, and carrying outtreatment after a reaction is completed to obtain a compound 5; and step 5, adding the compound 5 into hydrochloric acid, adjusting alkalinity after a reaction is completed, then adding Boc anhydride, carrying out stirring for a reaction, and carrying out treatment to obtain a compound 6.
Owner:SHANGHAI STA PHARMA R&D CO LTD
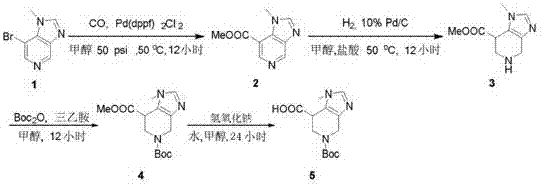
Synthetic method of 5-(boc t-butoxycarbonyl)-1-methyl-imidazopyridine-7-carboxylic acid
InactiveCN107188891AReasonable reaction process designMethod route shortOrganic chemistryChemical reactionCarboxylic acid
The invention relates to a synthetic method of 5-(boc t-butoxycarbonyl)-1-methyl-4,5,6,7-tetrahydro-1H-imidazo-[4,5-c]pyridine-7-carboxylic acid, and mainly aims to solve the technical problem that a method suitable for industrial synthesis is unavailable at present. The synthetic method comprises the four steps: I, performing a carbonyl inserting reaction under the catalysis of palladium to obtain a compound 2; II, hydrogenating the compound 2 under the catalysis of strong hydrochloric acid to obtain a compound 3; III, reacting the compound 3 with BOC acid anhydride under a triethylamine alkaline condition to obtain a compound 4; IV, hydrolyzing the compound 4 with sodium hydroxide to obtain a compound 5. The chemical reaction formula is shown in the description.
Owner:WUXI APPTEC (TIANJIN) CO LTD
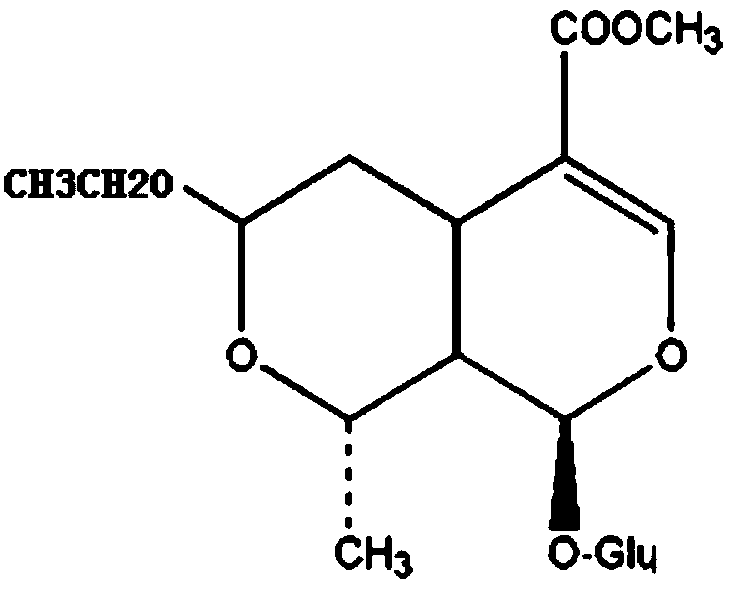
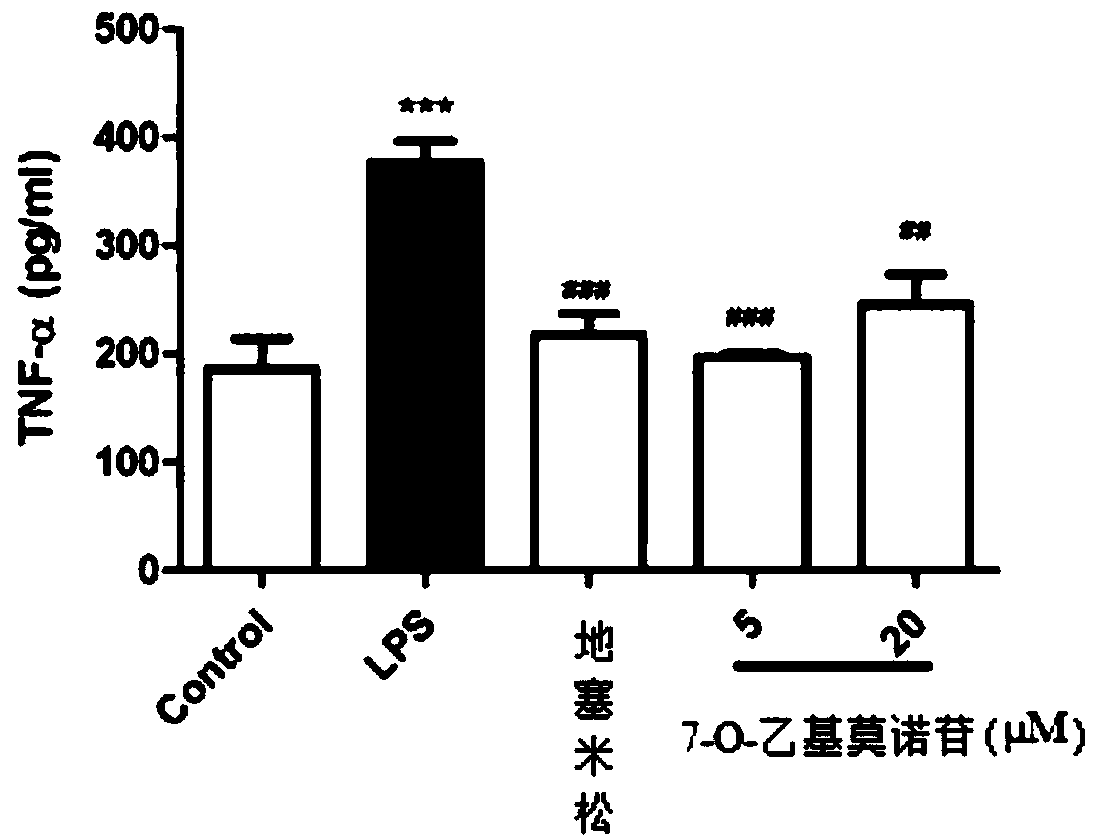
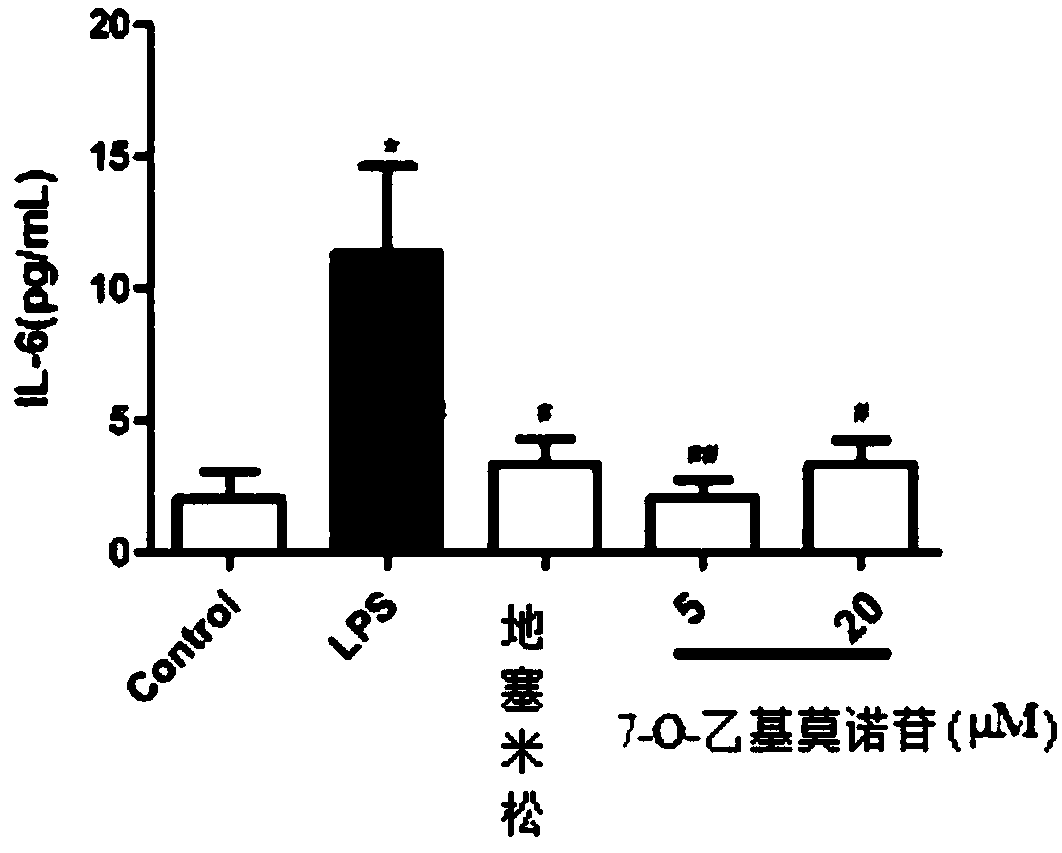
The preparation method of 7-o-ethyl morroniside
ActiveCN105968150BHigh synthesis rateLess impuritiesSugar derivativesAntipyreticEthyl groupAntiinflammatory drug
The invention discloses a preparation method of 7-O-ethyl morroniside. The best preparation process of 7-O-ethyl morroniside was screened out through a large number of experiments, including dissolution method, reaction pH value, heating temperature, heating time and other parameters, and the best purification method was optimized, and the entire reaction process design Reasonable, strong operability, high synthesis rate of 7‑O‑ethyl morroniside, less impurities, high purity, low cost, and industrial production can be realized. The present invention is further screened through in vivo and in vitro anti-inflammatory experiments, and the results show that 7-O-ethyl morroniside has good anti-inflammatory effects both in vivo and in vitro, and has the potential use of being prepared as an anti-inflammatory drug.
Owner:JIANGSU PROVINCIAL HOSPITAL OF TCM
Preparation method of Boc-1-(benzyl)-3-iodo-[1,8]diazaspiro[4,5]heptane-8-carboxylate
InactiveCN110183448AReasonable reaction process designShort routeOrganic chemistrySolventCarboxylate
The invention relates to a synthesis method of tert-butoxycarbonyl-1-(benzyl)-3-iodo-[1,8]diazaspiro[4,5]heptane-8-carboxylate, and mainly solve the technical problem that no synthesis method suitablefor industrialization exists at present. The method is divided into three steps: the first step, a compound 1 and benzylamine are reacted to obtain a compound 2 in the solvent toluene under the action of potassium carbonate; the second step, the compound 2 and allyl magnesium bromide are reacted to obtain a compound 3 in toluene; and the third step, the compound 3 and iodine are reacted to obtaina final compound 4 in the solvent acetonitrile under the action of sodium bicarbonate. The reaction formula is as shown in the specification.
Owner:WUXI APPTEC (TIANJIN) CO LTD
Synthesis method of 1-oxo-2,6-diazaspiro[3.5]nonane-6-tert-butyl formate
InactiveCN113214257AReasonable reaction process designShort synthetic routeOrganic chemistryBulk chemical productionNonaneButyl formate
The invention discloses a synthesis method of 1-oxo-2,6-diazaspiro[3.5]nonane-6-tert-butyl formate. The synthesis method comprises the following steps: in a first reaction solvent and an inert atmosphere, reacting a compound 2 with a compound 1 under the action of alkali to obtain a compound 3; and 2, removing a benzyl protecting group from the compound 3 to obtain a compound 4. The disclosed method has the advantages of easily available raw materials, convenience in operation, short route, high total yield, suitability for industrial production and the like, and mainly solves the technical problem that no suitable industrial synthesis method exists at present.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
Method for preparing tiotropium bromide
The invention discloses a method for preparing tiotropium bromide, which comprises the following steps of: preparing scopine-2,2-dithienyl glycolate from scopine and methyl 2,2-dithienyl glycolate, reacting the scopine-2,2-dithienyl glycolate with methyl bromide to prepare a tiotropium bromide crude product, and refining the tiotropium bromide crude product to obtain a tiotropium bromide finishedproduct. The method is characterized in that: the scopine and the methyl 2,2-dithienyl glycolate undergo ester exchange reaction under the action of dimethylbenzene, and the mixed catalysts of sodiumand sodium methoxide, and after the reaction, reaction solution is post-treated to obtain the scopine-2,2-dithienyl glycolate. In the method, in the process of preparing the scopine-2,2-dithienyl glycolate, the sodium and the sodium methoxide are simultaneously taken as the catalysts, and scopine isomer content after the reaction is less than 0.1 percent which completely meets specifications in the trial standards of European pharmacopoeia; and the method solves a big problem for the conventional preparation of the tiotropium bromide and is easy to realize industrial production.
Owner:ANHUI DEXINJIA BIOPHARM
Preparation method of tert-butyl-8-oxo-2-azaspiro-[4.5] decane-2-formate
PendingCN111518015AReasonable reaction process designLower synthesis costOrganic chemistryFormic acidCombinatorial chemistry
The invention relates to a preparation method of tert-butyl-8-oxo-2-azaspiro-[4.5] decane-2-formate, and mainly aims to solve the technical problems of high raw material cost, difficulty in reaction control, inconvenience in experimental operation and the like in an existing synthesis process. According to the method, tert-butyl-8-oxo-2-azaspiro-[4.5] decane-2-formate is prepared from cheap and easily available 1, 4-dioxaspiro [4.5] decane-8-one as an initial raw material through four steps of reaction. The reaction formula is shown in the specification. The tert-butyl-8-oxo-2-azaspiro-[4.5] decane-2-formate obtained in the invention is a useful intermediate or product synthesized from a plurality of medicines.
Owner:SHANGHAI STA PHARMA R&D CO LTD +1
Synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane
ActiveCN106831774AReasonable reaction process designShort routeOrganic chemistry methodsTrifluoromethylPalladium on carbon
The invention relates to a synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane, aiming at mainly solving the technical problem that a suitable industrial synthesis method does not exist at present. The synthesis method is divided into six steps as follows: firstly, taking a compound 1 and oxalyl chloride to react to obtain a compound 2; then, hydrogenating by palladium on carbon to generate a compound 3; taking the compound 3 to react with BOC (Butyloxycarbonyl) acid anhydride under an alkaline condition to obtain a compound 4; taking the compound 4 and acrylonitrile to be subjected to alkylation reaction under the action of sodium ethoxide to obtain a compound 5; carrying out reduction and ring closure on the compound 5 under the action of Raney nickel to obtain a compound 6; reducing the compound 6 with borane-dimethylsulfide to obtain a final compound 7, wherein a reaction formula is shown in the description.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD +2
Synthesis method of octahydro-4A,8-epoxy pyrido [4,3-C]azepine-6(5H)-tert-butyl formate
The invention discloses a synthesis method of octahydro-4A,8-epoxy pyrido[4,3-C]azepine-6(5H)-tert-butyl formate. The synthesis method comprises the following steps: 1, reacting a compound 1 with a catalytic amount of osmium tetroxide and a first oxidant to obtain a compound 2; 2, oxidizing an alcoholic hydroxyl group into an aldehyde group by the compound 2 under the action of a second oxidant to obtain a compound 3; 3, subjecting the compound 3 and benzyl amine to reduction ammoniation in an inert atmosphere under the action of a first reducing agent to obtain a compound 4; 4, subjecting the compound 4 to catalytic hydrogenation through a palladium catalyst in the presence of Boc2O to obtain a compound 5; 5, reducing the compound 5 through a second reducing agent to obtain a compound 6; and 6, carrying out catalytic hydrogenation on the compound 6 under the action of a palladium catalyst to obtain a compound 7. The method disclosed by the invention has the advantages of easily available raw materials, convenience in operation, safety in reaction, suitability for amplification, short route, high total yield, suitability for industrial production and the like.
Owner:WUXI APPTEC +1
Preparation method of 2-(2-(tert-butyloxycarbonyl)-2-azaspiro [3.4] octane-5-yl) acetic acid
ActiveCN112552222AReasonable reaction process designShort routeOrganic chemistryBulk chemical productionCarbonyl groupSpiro compound
The invention discloses a preparation method of 2-(2-(tert-butyloxycarbonyl)-2-azaspiro [3.4] octane-5-yl) acetic acid. The preparation method comprises the following steps: dissolving a compound 1 and a compound 2 in 2-methyl tetrahydrofuran, dropwise adding lithium bis (trimethylsilyl) amide, and reacting to obtain a compound 3; dissolving the compound 3 into acetone, and reacting with sodium iodide to obtain a compound 4; dissolving the compound 4 in 2-methyl tetrahydrofuran, dropwise adding n-butyllithium, and reacting to obtain a compound 5; dissolving the compound 5 into 2-methyl tetrahydrofuran, then adding a compound 6, alkali and 1, 8-diazabicyclo undecene-7-ene, and reacting at room temperature to obtain a compound 7; dissolving the compound 7 into ethyl acetate, adding a catalyst, and reacting in hydrogen to obtain a compound 8; dissolving the compound 8 into tetrahydrofuran and water, adding alkali for hydrolysis to obtain a final compound 9, and continuously optimizing thestructure of a spiro compound to obtain the synthesis method suitable for industrial production.
Owner:南通药明康德医药科技有限公司
2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid
ActiveCN107383035BReasonable reaction process designThe method route is simpleOrganic chemistry methodsAcetic anhydrideLithium hydroxide
The invention relates to a preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid. The preparation method comprises 11 steps: carrying out esterification on a dicarboxylic acid piperidine compound 1 in thionyl chloride to obtain a diester compound 2; then reducing a pyridine ring by utilizing palladium carbon to obtain a compound 3; then taking the compound 3 to react with Boc acid anhydride to obtain a Boc protected compound 4; hydrolyzing the compound 4 by utilizing lithium hydroxide to obtain a dicarboxylic acid compound 5; carrying out ring closure in acetic anhydride to obtain a compound 6; carrying out reduction and ring opening by utilizing NaBH4 to obtain a mixture of position isomerism compounds 7A and 7B; carrying out the ring closure in the presence of iodomethane and potassium carbonate to generate position isomerism lactone 8A and 8B; step 8, reducing the 8A, obtained by column passing separation, by utilizing DIBAH (Diisobutylaluminum Hydride) to obtain a compound 9; carrying out Wittig reaction to obtain a compound 10; carrying out Michael addition under an alkaline condition to obtain a compound 11; finally, hydrolyzing the compound 11 under the alkaline condition to obtain a final compound.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD +3
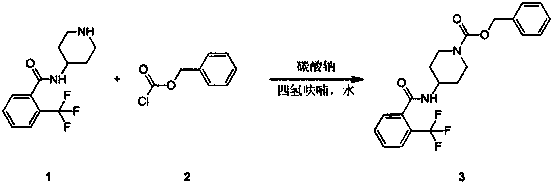
Synthesis method of nitrogen-(benzoxycarbonyl piperidine-4-yl)-2-(trifluoromethyl)benzamide
InactiveCN109503464AReasonable reaction process designAvoid postprocessingOrganic chemistrySynthesis methodsBenzyl chloroformate
The invention relates to a synthesis method of nitrogen-(benzoxycarbonyl piperidine-4-yl)-2-(trifluoromethyl)benzamide. The technical problem that the compound did not have the industrialized synthesis route report before is mainly solved. According to the technical scheme of the invention, the synthesis method of the nitrogen-(benzoxycarbonyl piperidine-4-yl)-2-(trifluoromethyl)benzamide comprises the following steps: adding nitrogen-(piperidine-4-yl)-2-(trifluoromethyl)benzamide and sodium carbonate into tetrahydrofuran and water, adding benzyl chloroformate slowly and dropwise, performing reaction, and performing aftertreatment to obtain the nitrogen-(benzoxycarbonyl piperidine-4-yl)-2-(trifluoromethyl)benzamide. The cheap and easily available raw materials are adopted, so that the complicated aftertreatment process is avoided; and the target molecule is obtained only through one-step reaction, so that amplification is facilitated.
Owner:WUXI APPTEC (TIANJIN) CO LTD
Preparation method of tert-butyl-1,8-dioxa-4,11-diazaspiro[5.6]dodecane-11-carboxylate
ActiveCN111548356BReasonable reaction process designMethod route shortOrganic chemistryBulk chemical productionBenzoic acidDodecane
The invention relates to a method for preparing tert-butyl-1,8-dioxa-4,11-diazaspiro[5.6]dodecane-11-carboxylate, which mainly solves the problem that there is currently no technology suitable for industrial synthesis methods question. The present invention is divided into six steps: the first step, first dissolving compound 1 in dichloromethane, then adding Boc anhydride, and reacting to obtain compound 2; the second step, compound 2 and sodium hydrogen, 3-chloro-2-chloromethyl ‑1‑propylene reaction to obtain compound 3; in the third step, compound 3 is dissolved in dichloromethane, and m-chloroperoxybenzoic acid is added to react to obtain compound 4; in the fourth step, compound 4 is reacted in 2‑benzylaminoethanol Compound 5 was obtained; in the fifth step, compound 5 was reacted with sodium hydrogen and p-toluenesulfonimidazole in tetrahydrofuran to obtain compound 6; in the sixth step, compound 6 was reacted with palladium hydroxide in methanol to obtain final compound 7.
Owner:WUXI APPTEC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate](https://images-eureka.patsnap.com/patent_img/c419c075-256f-4485-9366-6c39e9d8594c/204216DEST_PATH_IMAGE004.png)
![Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate Synthesis method of tert-butyl-1, 7-diazaspiro [3.5] nonane-1-formate](https://images-eureka.patsnap.com/patent_img/c419c075-256f-4485-9366-6c39e9d8594c/349392DEST_PATH_IMAGE002.png)
![Synthesis method of 8-oxo-5-oxa-2-diazaspiro [3.5] nonane-2-tert-butyl formate Synthesis method of 8-oxo-5-oxa-2-diazaspiro [3.5] nonane-2-tert-butyl formate](https://images-eureka.patsnap.com/patent_img/c2bc7891-b076-45ae-af8c-a87decde9903/388939DEST_PATH_IMAGE002.png)
![Synthesis method of 8-oxo-5-oxa-2-diazaspiro [3.5] nonane-2-tert-butyl formate Synthesis method of 8-oxo-5-oxa-2-diazaspiro [3.5] nonane-2-tert-butyl formate](https://images-eureka.patsnap.com/patent_img/c2bc7891-b076-45ae-af8c-a87decde9903/97897DEST_PATH_IMAGE002.png)
![Synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate Synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate](https://images-eureka.patsnap.com/patent_img/6d130abe-9731-4832-84f2-27c828084aaa/858410DEST_PATH_IMAGE002.png)
![Synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate Synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate](https://images-eureka.patsnap.com/patent_img/6d130abe-9731-4832-84f2-27c828084aaa/DEST_PATH_IMAGE002.png)
![Synthesis method of 7-hydroxymethyl-2,5-diazaspiro [3,4] octane-2-dimethylethylester Synthesis method of 7-hydroxymethyl-2,5-diazaspiro [3,4] octane-2-dimethylethylester](https://images-eureka.patsnap.com/patent_img/9922076c-4753-44af-97a5-80cae6f1cda4/DEST_PATH_IMAGE003.png)
![Synthetic method for tert-butyl 1,10-dioxo-2,7-diazaspiro[4.5]decane-7-carboxylate Synthetic method for tert-butyl 1,10-dioxo-2,7-diazaspiro[4.5]decane-7-carboxylate](https://images-eureka.patsnap.com/patent_img/123aef50-11a3-4b9c-87b3-d13af7b7691d/DEST_PATH_IMAGE004.png)
![Synthetic method for tert-butyl 1,10-dioxo-2,7-diazaspiro[4.5]decane-7-carboxylate Synthetic method for tert-butyl 1,10-dioxo-2,7-diazaspiro[4.5]decane-7-carboxylate](https://images-eureka.patsnap.com/patent_img/123aef50-11a3-4b9c-87b3-d13af7b7691d/100002_DEST_PATH_IMAGE002.png)
![6-(tert-butoxycarbonyl) octahydro-furo [2,3-c] pyridine-4-carboxylic acid synthetic method 6-(tert-butoxycarbonyl) octahydro-furo [2,3-c] pyridine-4-carboxylic acid synthetic method](https://images-eureka.patsnap.com/patent_img/1dd80e21-37bd-4568-95b9-0b806df75286/695976DEST_PATH_IMAGE001.PNG)
![6-(tert-butoxycarbonyl) octahydro-furo [2,3-c] pyridine-4-carboxylic acid synthetic method 6-(tert-butoxycarbonyl) octahydro-furo [2,3-c] pyridine-4-carboxylic acid synthetic method](https://images-eureka.patsnap.com/patent_img/1dd80e21-37bd-4568-95b9-0b806df75286/938519DEST_PATH_IMAGE001.PNG)
![Synthesis method of cis5-tert-butyl-3A-methyl-tetrahydro 1H-furan[3,4 c] pyrrole 3A,5(3H) dicarboxylic ester Synthesis method of cis5-tert-butyl-3A-methyl-tetrahydro 1H-furan[3,4 c] pyrrole 3A,5(3H) dicarboxylic ester](https://images-eureka.patsnap.com/patent_img/1b155c81-c2c1-47f6-ba38-9872cf0937bd/240257DEST_PATH_IMAGE002.png)
![Synthesis method of cis5-tert-butyl-3A-methyl-tetrahydro 1H-furan[3,4 c] pyrrole 3A,5(3H) dicarboxylic ester Synthesis method of cis5-tert-butyl-3A-methyl-tetrahydro 1H-furan[3,4 c] pyrrole 3A,5(3H) dicarboxylic ester](https://images-eureka.patsnap.com/patent_img/1b155c81-c2c1-47f6-ba38-9872cf0937bd/577916DEST_PATH_IMAGE002.png)
![Synthesis method of 8-(tert-butyloxycarbonyl)-6, 7, 8, 9-tetrahydro-5-hydro-imidazole[1, 5-a][1, 4]diaza-6-carboxylic acid Synthesis method of 8-(tert-butyloxycarbonyl)-6, 7, 8, 9-tetrahydro-5-hydro-imidazole[1, 5-a][1, 4]diaza-6-carboxylic acid](https://images-eureka.patsnap.com/patent_img/1e4c0621-3f13-421a-91d2-a6dbb3fcd00d/111152DEST_PATH_IMAGE002.PNG)
![Synthesis method of 8-(tert-butyloxycarbonyl)-6, 7, 8, 9-tetrahydro-5-hydro-imidazole[1, 5-a][1, 4]diaza-6-carboxylic acid Synthesis method of 8-(tert-butyloxycarbonyl)-6, 7, 8, 9-tetrahydro-5-hydro-imidazole[1, 5-a][1, 4]diaza-6-carboxylic acid](https://images-eureka.patsnap.com/patent_img/1e4c0621-3f13-421a-91d2-a6dbb3fcd00d/234463DEST_PATH_IMAGE001.PNG)
![Synthesis method of 8-(tert-butyloxycarbonyl)-6, 7, 8, 9-tetrahydro-5-hydro-imidazole[1, 5-a][1, 4]diaza-6-carboxylic acid Synthesis method of 8-(tert-butyloxycarbonyl)-6, 7, 8, 9-tetrahydro-5-hydro-imidazole[1, 5-a][1, 4]diaza-6-carboxylic acid](https://images-eureka.patsnap.com/patent_img/1e4c0621-3f13-421a-91d2-a6dbb3fcd00d/255377DEST_PATH_IMAGE007.PNG)



![Synthesis method of tert butyl-1-(hydroxymethyl)-3-oxa-9-azaspiro-[5.5] undecane-9-formyl ester Synthesis method of tert butyl-1-(hydroxymethyl)-3-oxa-9-azaspiro-[5.5] undecane-9-formyl ester](https://images-eureka.patsnap.com/patent_img/81ec9440-aefb-435f-b353-3603c2ed7be1/124493DEST_PATH_IMAGE002.png)
![Synthesis method of tert butyl-1-(hydroxymethyl)-3-oxa-9-azaspiro-[5.5] undecane-9-formyl ester Synthesis method of tert butyl-1-(hydroxymethyl)-3-oxa-9-azaspiro-[5.5] undecane-9-formyl ester](https://images-eureka.patsnap.com/patent_img/81ec9440-aefb-435f-b353-3603c2ed7be1/DEST_PATH_IMAGE001.png)
![Synthesis method of spiro[2.5]octane-5-carboxylic acid Synthesis method of spiro[2.5]octane-5-carboxylic acid](https://images-eureka.patsnap.com/patent_img/b88b1387-7bd8-4b55-a64c-8b2428bc1236/201310047662X100001DEST_PATH_IMAGE001.png)
![Synthesis method of spiro[2.5]octane-5-carboxylic acid Synthesis method of spiro[2.5]octane-5-carboxylic acid](https://images-eureka.patsnap.com/patent_img/b88b1387-7bd8-4b55-a64c-8b2428bc1236/4207DEST_PATH_IMAGE005.png)
![Synthesis method of spiro[2.5]octane-5-carboxylic acid Synthesis method of spiro[2.5]octane-5-carboxylic acid](https://images-eureka.patsnap.com/patent_img/b88b1387-7bd8-4b55-a64c-8b2428bc1236/165695DEST_PATH_IMAGE005.png)
![Preparation method of 4-methyl formate-2-oxo-1,8-diazaspiro[4.5]decane-8-tert-Butyl formate Preparation method of 4-methyl formate-2-oxo-1,8-diazaspiro[4.5]decane-8-tert-Butyl formate](https://images-eureka.patsnap.com/patent_img/036da539-82b9-4b8d-b27b-d6eb8b6e883b/607281DEST_PATH_IMAGE002.png)
![Preparation method of 4-methyl formate-2-oxo-1,8-diazaspiro[4.5]decane-8-tert-Butyl formate Preparation method of 4-methyl formate-2-oxo-1,8-diazaspiro[4.5]decane-8-tert-Butyl formate](https://images-eureka.patsnap.com/patent_img/036da539-82b9-4b8d-b27b-d6eb8b6e883b/DEST_PATH_IMAGE001.png)
![Synthetic method of 6-oxo-2-azaspiro[3,3]heptane-2-t-butyl carboxylate Synthetic method of 6-oxo-2-azaspiro[3,3]heptane-2-t-butyl carboxylate](https://images-eureka.patsnap.com/patent_img/1fb6fac1-7a0b-46a5-80fc-1ca1f4c59ece/BDA0000119406130000011.PNG)
![Synthetic method of 6-oxo-2-azaspiro[3,3]heptane-2-t-butyl carboxylate Synthetic method of 6-oxo-2-azaspiro[3,3]heptane-2-t-butyl carboxylate](https://images-eureka.patsnap.com/patent_img/1fb6fac1-7a0b-46a5-80fc-1ca1f4c59ece/BDA0000119406130000012.PNG)
![Synthetic method of 6-oxo-2-azaspiro[3,3]heptane-2-t-butyl carboxylate Synthetic method of 6-oxo-2-azaspiro[3,3]heptane-2-t-butyl carboxylate](https://images-eureka.patsnap.com/patent_img/1fb6fac1-7a0b-46a5-80fc-1ca1f4c59ece/BDA0000119406130000021.PNG)
![Method for preparing 5-(tert-butoxycarbonyl)-2-oxooctahydropyrrolo[3, 4-b]pyrrole-3a-carboxylic acid Method for preparing 5-(tert-butoxycarbonyl)-2-oxooctahydropyrrolo[3, 4-b]pyrrole-3a-carboxylic acid](https://images-eureka.patsnap.com/patent_img/42fd5b46-df57-4fae-97ec-b587c98d7d43/DEST_PATH_IMAGE003.png)
![Synthetic method for 1-(ethoxycarbonyl)imidazo[1,5]pyridine-6-carboxylic acid Synthetic method for 1-(ethoxycarbonyl)imidazo[1,5]pyridine-6-carboxylic acid](https://images-eureka.patsnap.com/patent_img/90380351-ece5-42d0-8697-5638b1785685/32356DEST_PATH_IMAGE003.png)
![Synthetic method for 1-(ethoxycarbonyl)imidazo[1,5]pyridine-6-carboxylic acid Synthetic method for 1-(ethoxycarbonyl)imidazo[1,5]pyridine-6-carboxylic acid](https://images-eureka.patsnap.com/patent_img/90380351-ece5-42d0-8697-5638b1785685/551434DEST_PATH_IMAGE002.png)
![Synthesis method of 1,7-diazaspiro[3.5]nonane-7-tert-butyl formate Synthesis method of 1,7-diazaspiro[3.5]nonane-7-tert-butyl formate](https://images-eureka.patsnap.com/patent_img/c592fcaf-d14a-4b91-973d-4425df5d7b93/FDA0003032556600000011.png)
![Synthesis method of 1,7-diazaspiro[3.5]nonane-7-tert-butyl formate Synthesis method of 1,7-diazaspiro[3.5]nonane-7-tert-butyl formate](https://images-eureka.patsnap.com/patent_img/c592fcaf-d14a-4b91-973d-4425df5d7b93/BDA0003032556610000021.png)
![Synthesis method of 1,7-diazaspiro[3.5]nonane-7-tert-butyl formate Synthesis method of 1,7-diazaspiro[3.5]nonane-7-tert-butyl formate](https://images-eureka.patsnap.com/patent_img/c592fcaf-d14a-4b91-973d-4425df5d7b93/BDA0003032556610000041.png)
![Preparation method of tert-butyl-1-oxo-6-oxa-9-azaspiro[4.5]decane-9-carboxylate Preparation method of tert-butyl-1-oxo-6-oxa-9-azaspiro[4.5]decane-9-carboxylate](https://images-eureka.patsnap.com/patent_img/e2822d84-7891-4b7b-9362-44a4c44496a9/222973DEST_PATH_IMAGE002.png)
![Preparation method of Boc-1-(benzyl)-3-iodo-[1,8]diazaspiro[4,5]heptane-8-carboxylate Preparation method of Boc-1-(benzyl)-3-iodo-[1,8]diazaspiro[4,5]heptane-8-carboxylate](https://images-eureka.patsnap.com/patent_img/0229c71d-9623-4421-9b48-ec93bcf74223/DEST_PATH_IMAGE002.png)
![Preparation method of Boc-1-(benzyl)-3-iodo-[1,8]diazaspiro[4,5]heptane-8-carboxylate Preparation method of Boc-1-(benzyl)-3-iodo-[1,8]diazaspiro[4,5]heptane-8-carboxylate](https://images-eureka.patsnap.com/patent_img/0229c71d-9623-4421-9b48-ec93bcf74223/DEST_PATH_IMAGE004.png)
![Synthesis method of 1-oxo-2,6-diazaspiro[3.5]nonane-6-tert-butyl formate Synthesis method of 1-oxo-2,6-diazaspiro[3.5]nonane-6-tert-butyl formate](https://images-eureka.patsnap.com/patent_img/088bb8cf-1d65-4e7e-becd-3d3bce0e9e73/FDA0003032558870000011.png)
![Synthesis method of 1-oxo-2,6-diazaspiro[3.5]nonane-6-tert-butyl formate Synthesis method of 1-oxo-2,6-diazaspiro[3.5]nonane-6-tert-butyl formate](https://images-eureka.patsnap.com/patent_img/088bb8cf-1d65-4e7e-becd-3d3bce0e9e73/BDA0003032558880000011.png)
![Synthesis method of 1-oxo-2,6-diazaspiro[3.5]nonane-6-tert-butyl formate Synthesis method of 1-oxo-2,6-diazaspiro[3.5]nonane-6-tert-butyl formate](https://images-eureka.patsnap.com/patent_img/088bb8cf-1d65-4e7e-becd-3d3bce0e9e73/BDA0003032558880000031.png)
![Preparation method of tert-butyl-8-oxo-2-azaspiro-[4.5] decane-2-formate Preparation method of tert-butyl-8-oxo-2-azaspiro-[4.5] decane-2-formate](https://images-eureka.patsnap.com/patent_img/15b81347-2bcc-4abe-b451-3130dd7b24a8/DEST_PATH_IMAGE002.png)
![Preparation method of tert-butyl-8-oxo-2-azaspiro-[4.5] decane-2-formate Preparation method of tert-butyl-8-oxo-2-azaspiro-[4.5] decane-2-formate](https://images-eureka.patsnap.com/patent_img/15b81347-2bcc-4abe-b451-3130dd7b24a8/DEST_PATH_IMAGE004.png)
![Preparation method of tert-butyl-8-oxo-2-azaspiro-[4.5] decane-2-formate Preparation method of tert-butyl-8-oxo-2-azaspiro-[4.5] decane-2-formate](https://images-eureka.patsnap.com/patent_img/15b81347-2bcc-4abe-b451-3130dd7b24a8/DEST_PATH_IMAGE006.png)
![Synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane Synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane](https://images-eureka.patsnap.com/patent_img/1b74b72f-deec-4a20-bc0c-0c6d6b6c9213/BSA0000140068570000011.png)
![Synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane Synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane](https://images-eureka.patsnap.com/patent_img/1b74b72f-deec-4a20-bc0c-0c6d6b6c9213/BSA0000140068570000021.png)
![Synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane Synthesis method of (6S,7S)-9-t-butyloxycarbonyl-7-(trifluoromethyl)-2,9-diazaspiro[5.5]undecane](https://images-eureka.patsnap.com/patent_img/1b74b72f-deec-4a20-bc0c-0c6d6b6c9213/FSA0000140068560000011.png)
![Synthesis method of octahydro-4A,8-epoxy pyrido [4,3-C]azepine-6(5H)-tert-butyl formate Synthesis method of octahydro-4A,8-epoxy pyrido [4,3-C]azepine-6(5H)-tert-butyl formate](https://images-eureka.patsnap.com/patent_img/211d78b1-c712-4eb3-9196-cf8924d956a1/FDA0003032693610000011.png)
![Synthesis method of octahydro-4A,8-epoxy pyrido [4,3-C]azepine-6(5H)-tert-butyl formate Synthesis method of octahydro-4A,8-epoxy pyrido [4,3-C]azepine-6(5H)-tert-butyl formate](https://images-eureka.patsnap.com/patent_img/211d78b1-c712-4eb3-9196-cf8924d956a1/BDA0003032693620000021.png)
![Synthesis method of octahydro-4A,8-epoxy pyrido [4,3-C]azepine-6(5H)-tert-butyl formate Synthesis method of octahydro-4A,8-epoxy pyrido [4,3-C]azepine-6(5H)-tert-butyl formate](https://images-eureka.patsnap.com/patent_img/211d78b1-c712-4eb3-9196-cf8924d956a1/BDA0003032693620000051.png)
![Preparation method of 2-(2-(tert-butyloxycarbonyl)-2-azaspiro [3.4] octane-5-yl) acetic acid Preparation method of 2-(2-(tert-butyloxycarbonyl)-2-azaspiro [3.4] octane-5-yl) acetic acid](https://images-eureka.patsnap.com/patent_img/22aae465-51fa-4709-a1c0-f4bdd1cb4d17/FDA0002844708750000011.png)
![Preparation method of 2-(2-(tert-butyloxycarbonyl)-2-azaspiro [3.4] octane-5-yl) acetic acid Preparation method of 2-(2-(tert-butyloxycarbonyl)-2-azaspiro [3.4] octane-5-yl) acetic acid](https://images-eureka.patsnap.com/patent_img/22aae465-51fa-4709-a1c0-f4bdd1cb4d17/FDA0002844708750000021.png)
![Preparation method of 2-(2-(tert-butyloxycarbonyl)-2-azaspiro [3.4] octane-5-yl) acetic acid Preparation method of 2-(2-(tert-butyloxycarbonyl)-2-azaspiro [3.4] octane-5-yl) acetic acid](https://images-eureka.patsnap.com/patent_img/22aae465-51fa-4709-a1c0-f4bdd1cb4d17/BDA0002844708760000011.png)
![2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid 2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid](https://images-eureka.patsnap.com/patent_img/2678f52b-b095-46d3-91e6-aa4bbbe3a737/DEST_PATH_IMAGE003.png)
![2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid 2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid](https://images-eureka.patsnap.com/patent_img/2678f52b-b095-46d3-91e6-aa4bbbe3a737/505010DEST_PATH_IMAGE004.png)
![2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid 2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid](https://images-eureka.patsnap.com/patent_img/2678f52b-b095-46d3-91e6-aa4bbbe3a737/600639DEST_PATH_IMAGE002.png)
![Preparation method of tert-butyl-1,8-dioxa-4,11-diazaspiro[5.6]dodecane-11-carboxylate Preparation method of tert-butyl-1,8-dioxa-4,11-diazaspiro[5.6]dodecane-11-carboxylate](https://images-eureka.patsnap.com/patent_img/2fef35fc-79ca-45e4-9203-92bc9125fe1f/734077DEST_PATH_IMAGE004.png)