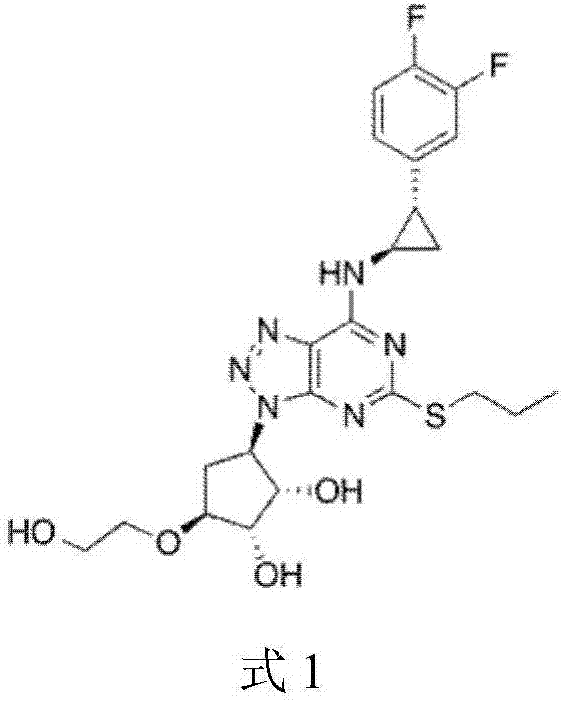
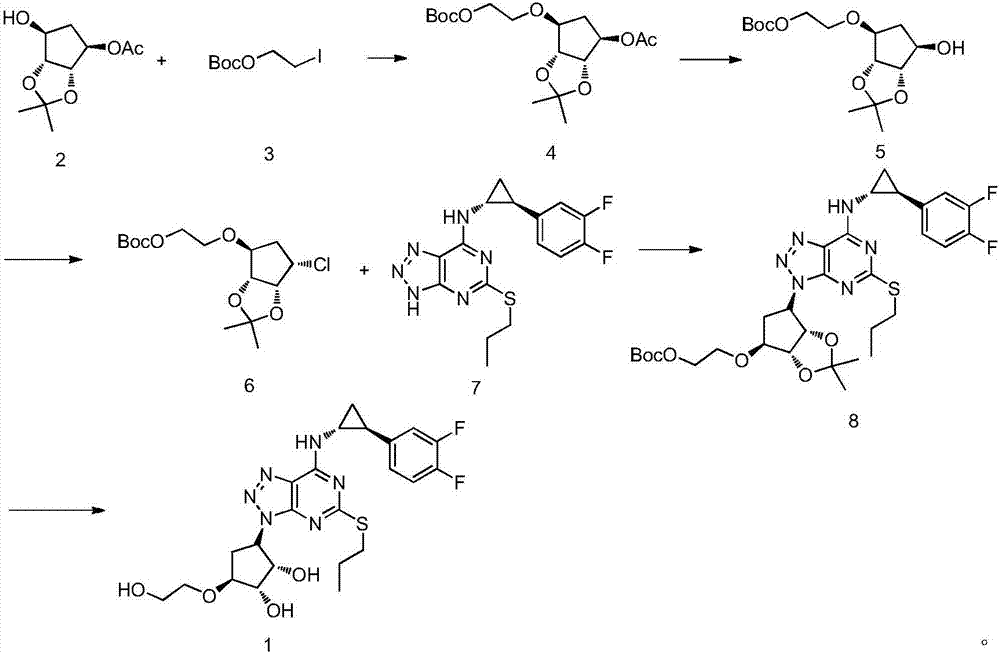
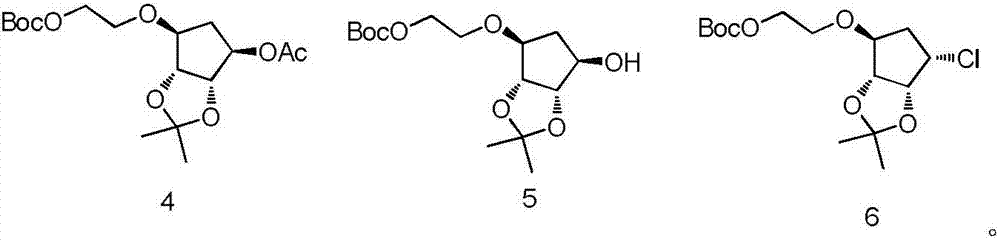
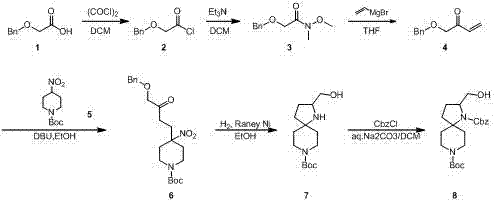
Patents
Literature
32results about How to "The reaction is easy to scale up" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method for bicyclo [1.1.1] pentane-1,3-dicarboxylic acid dimethylester
InactiveCN105294442AMethod route shortHigh yieldPreparation from carboxylic acid halidesMeth-Combinatorial chemistry
The invention relates to a preparation method for bicyclo [1.1.1] pentane-1,3-dicarboxylic acid dimethylester, and mainly solves the technical problem that a method suitable for industrial synthesis does not exist at present. The preparation method comprises the following six steps: firstly, reacting a compound (1) and bromoform under an alkaline condition to obtain a compound (2); secondly, reacting the compound (2) and lithium methide to obtain a compound (3); thirdly, illuminating the compound (3) and diacetyl by a high-pressure mercury lamp to obtain a compound (4); fourthly, treating with sodium hypochlorite to obtain a compound (5); fifthly, reacting the compound (5) and thionyl chloride to obtain a compound (6); and finally, treating the compound (6) under the effect of methanol to obtain a compound (7) which is a final product. A reaction formula is as shown in the specification, and the acquired bicyclo [1.1.1] pentane-1,3-dicarboxylic acid dimethylester is a useful midbody or product for synthesis of many drugs.
Owner:SHANGHAI STA PHARMA R&D CO LTD +3
Synthesis method for 4-(tert-butoxycarbonyl) octahydrofuro[3,2-b] pyridine-6-carboxylic acid
ActiveCN105418620AReduce pollutionMethod route shortOrganic chemistryFuranTert-Butyloxycarbonyl protecting group
The present incention relates to a synthesis method for 4-(tert-butoxycarbonyl) octahydrofuro[3,2-b] pyridine-6-carboxylic acid, and mainly solves the technical problem of no synthesis method suitable for industrialization at present. The synthesis method comprises six steps: first esterifying a compound 1 to obtain a compound 2; then performing iodination to produce a compound 3; acetylating the compound 3 to obtain a compound 4; then performing coupling to obtain a compound 5; performing cyclization to obtain a compound 6; performing hydrogenation to obtain a compound 7, then reacting with Boc anhydride to obtain a compound 8; and performing hydrogenation to obtain a final compound 9. The reaction formula is shown in the description.
Owner:WUXI APPTEC (TIANJIN) CO LTD
Synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate
InactiveCN109503605AReasonable reaction process designThe method route is simpleOrganic chemistrySynthesis methodsEthyl acetate
The invention relates to a synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate. The technical problem that an appropriate industrial synthesis method is inexistent at presentis mainly solved. The synthesis method is divided into two steps: the first step, adding triethylamine in a methanol solution dissolved with a compound 1 and amino ethyl acetate hydrochloride, and then dropwise adding tetraisopropyl titanate in the reaction solution, after completing the dropwise adding, reacting the mixed system for 12 h at 25 DEG C; adding trimethylsilyl cyanide in the reactionsystem at 25 DEG C, and then reacting for 12 h at 25 DEG C, performing post-treatment to obtain a compound 2; and the second step, adding the compound 2, nickel and ammonia water in the methanol solution, and performing hydrogenation to obtain the final compound.
Owner:上海合全医药有限公司
4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method
InactiveCN105669687AMethod route shortHigh yieldOrganic chemistryFuranTert-Butyloxycarbonyl protecting group
The present invention relates to a 4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method and mainly solves a technical problem that currently no suitable industrial synthetic method exists. The synthetic method comprises the following four steps: firstly, a compound 1 is subjected to an alkylation reaction with bromo chloroethane to obtain a compound 2; then the compound 2 is subjected to an intra-molecular cyclization reaction to obtain a compound 3; the compound 3 is subjected to a double bond hydrogenation reduction to obtain a compound 4; and the compound 4 is hydrolyzed to obtain a compound 5. The response equations are described as follows.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
Method for synthesizing compound ticagrelor and synthesized intermediate thereby
ActiveCN107513070AMild reaction conditionsSimple reaction conditionsOrganic chemistryTicagrelorCombinatorial chemistry
The invention discloses a method for synthesizing a compound ticagrelor and a synthesized intermediate thereby. The method comprises the following steps: carrying out a substitution reaction between a compound (2) and a compound (3) to prepare a compound (4); carrying out a reduction reaction on the compound (4) to prepare a compound (5); carrying out a chlorination reaction on the compound (5) to prepare a compound (6); carrying out a substitution reaction between the compound (6) and a compound (7) to prepare a compound (8); and finally, carrying out a hydroxyl deprotection reaction on the compound (8), thereby obtaining the ticagrelor (1). The reaction formulas are as shown in the specification. The invention provides a novel method for synthesizing ticagrelor. The synthetic method has the advantages of novel technical route, simple and convenient operation, high synthesis yield, high product purity, cheap and readily available raw materials and the like, and is suitable for industrialized production. Meanwhile, the synthesized ticagrelor intermediate provides a novel intermediate raw material for preparation of the ticagrelor.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
6-tert-butyloxycarbonyl octahydro-2H-pyran[3,2-c]pyridine-8-carboxylic acid synthesis method
ActiveCN105601639AMethod route shortHigh yieldOrganic chemistryTert-Butyloxycarbonyl protecting groupCarboxylic acid
The present invention relates to a 6-tert-butyloxycarbonyl octahydro-2H-pyran[3,2-c]pyridine-8-carboxylic acid synthesis method. In the prior art, the suitable industrial synthesis method does not exist. A purpose of the present invention is to mainly solve the technical problem in the prior art. The synthesis method comprises six steps and specifically comprises that a compound 1 and 1-bromo-3-chloro-propane are subjected to an alkylation reaction to obtain a compound 2, an intramolecular cyclization reaction is performed to generate a compound 3, the double bond of the compound 3 is subjected to hydrogenation reduction to obtain a compound 4, and the compound 4 is subjected to hydrolysis to obtain a compound. The reaction formula is defined in the specification.
Owner:SHANGHAI STA PHARMA R&D CO LTD +1
Method for preparing 5-(tert-butoxycarbonyl)-2-oxooctahydropyrrolo[3, 4-b]pyrrole-3a-carboxylic acid
InactiveCN109503593AReasonable reaction process designThe method route is simpleOrganic chemistry methodsSodium bicarbonateTert-Butyloxycarbonyl protecting group
The invention relates to a method for synthesizing (3aS, 6aR)-5-(tert-butoxycarbonyl)-2-oxooctahydropyrrolo[3, 4-b]pyrrole-3a-carboxylic acid, and mainly solves a technical problem of absence of a method suitable for industrial synthesis at present. The method provided by the invention comprises the following four steps: step one, dissolving a compound 1, ethyl bromoacetate and tetrabutylammoniumfluoride in tetrahydrofuran to obtain a compound 2; step two, dissolving the compound 2, hydroxylamine hydrochloride and sodium bicarbonate in a mixed solvent of tetrahydrofuran and ethanol to obtaina compound 3; step three, adding the compound 3, Raney nickel and ammonia water into ethanol, filtering and spin-drying to obtain a compound 4 after finishing reaction; and step four, adding the compound 4 and sodium ethoxide into ethanol, performing reflux reaction to obtain a white solid compound 5, namely, (3aS, 6aR)-5-(tert-butoxycarbonyl)-2-oxooctahydropyrrolo[3, 4-b]pyrrole-3a-carboxylic acid.
Owner:武汉药明康德新药开发有限公司
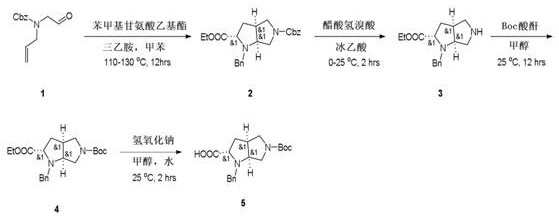
A preparation method of 4,5-dihydro-1h,3h-pyrrolo[1,2-a][1,4]diazepine-2,4-dicarboxylate-2-tert-butyl ester
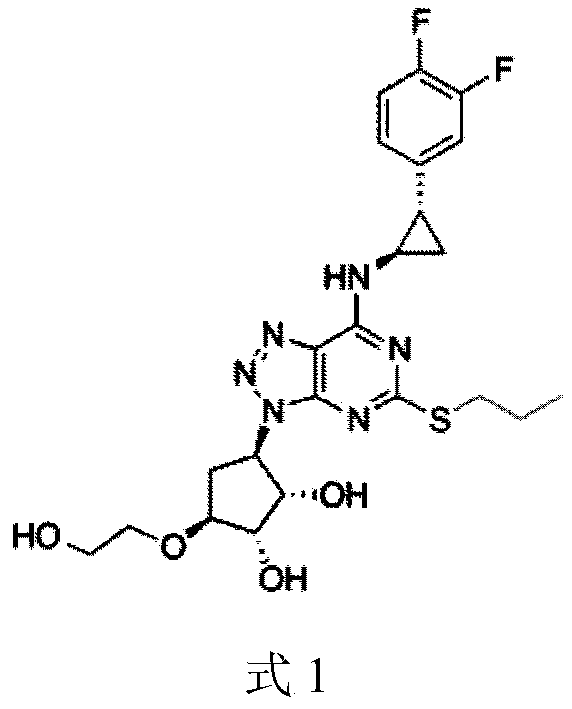
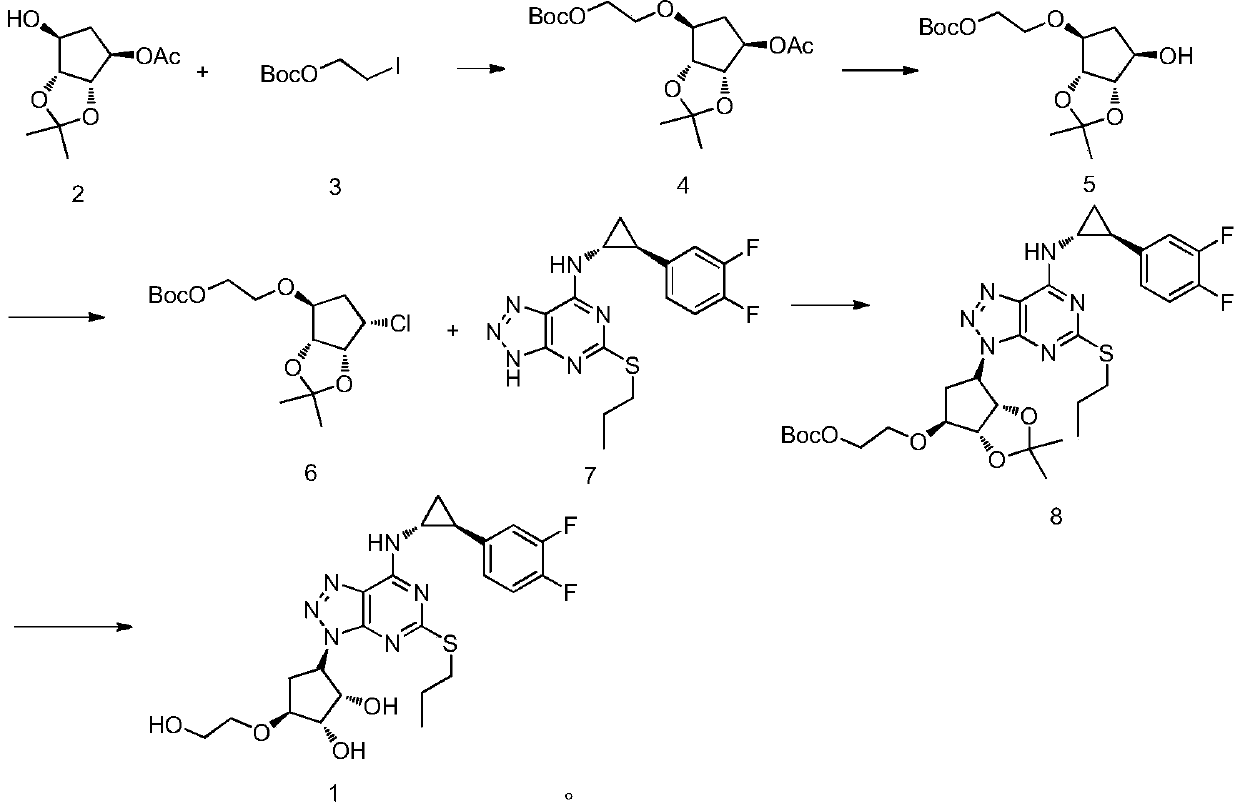
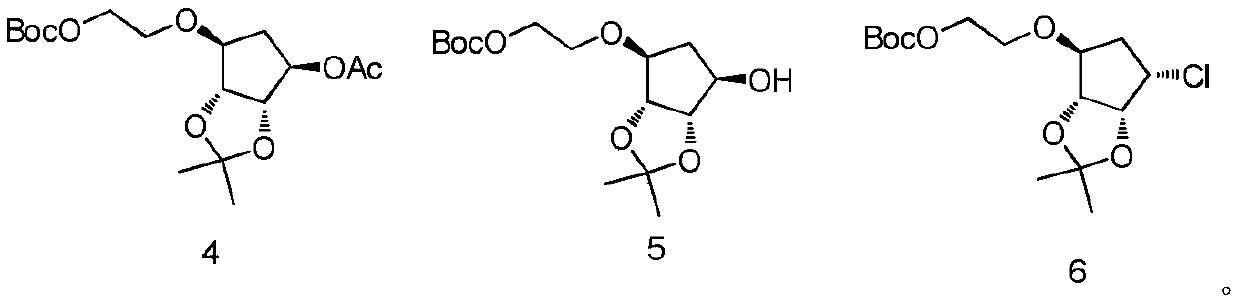
ActiveCN110551129BShort synthetic routeThe reaction is easy to scale upOrganic chemistryBulk chemical productionTert butylPharmaceutical Substances
The present invention relates to a kind of 4,5-dihydro-1H,3H-pyrrolo[1,2-A][1,4]diazepine-2,4-dicarboxylic acid-2-tert-butyl ester The preparation method mainly solves the technical problem that there is no suitable industrial synthesis method at present. The present invention is divided into five steps. First, compound 1 is dissolved in acetonitrile and reacts with compound 2 at room temperature under the action of potassium carbonate to generate compound 3. The second step is the reductive amination of compound 2 and benzylamine in ethanol by adding sodium borohydride and simultaneous ring closure to obtain compound 4. Then in compound 4 ethanol solution, add 10% palladium carbon hydrogen to remove the benzyl protecting group of compound 4 to obtain compound 5, then in compound 5 methanol solution, pass through and BOC 2 O is reacted to obtain compound 6, and finally compound 6 is hydrolyzed by adding sodium hydroxide in a mixed solvent of methanol and water to obtain final product 7. The compound obtained in the present invention is a useful intermediate or product for the synthesis of many medicines.
Owner:CHANGZHOU HEQUAN PHARMA CO LTD
Preparation method of tert-butyl 2-iodomethyl-1-oxa-7-azaspirane [3,5] nonane-7-carboxylic ester
The invention relates to a preparation method of tert-butyl 2-iodomethyl-1-oxa-7-azaspirane [3,5] nonane-7-carboxylic ester and mainly solves the technical problem that no appropriate industrial synthesis method exists at present. The method comprises two steps as follows: firstly, a compound 1 and a compound 2 produce a compound 3 under the action of zinc powder and ammonium chloride, then the compound 3 reacts with iodine and sodium bicarbonate in acetonitrile, a compound 4 is obtained, and the equation is shown in the specification. The compound obtained with the method is a useful intermediate for synthesis of numerous drugs or product.
Owner:上海药明康德新药开发有限公司 +2
A kind of preparation method of 3,3'-bisindole compound
InactiveCN105669516BEasy to separate and purifyHigh yieldOrganic chemistryCombinatorial chemistrySolvent
Owner:SUN YAT SEN UNIV
Preparation method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid
The invention relates to a synthesis method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid, aiming at mainly solving the technical problem that a suitable industrial synthesis method does not exist at present. The synthesis method is divided into four steps as follows: firstly, taking a compound 1 to react with n-butyl lithium and benzyl chloroformate in tetrahydrofuran to obtain a compound 2; then, taking the compound 2 to react with diphenyl chlorophosphate and lithium hexamethyldisilazide to obtain a compound 3; inserting carbonyl into the compound 3 in the presence of CO under the action of palladium acetate and triphenylphosphine to obtain a compound 4; under the action of hydrogen and palladium on carbon, carrying out hydrogenation reduction again to obtain a target compound 5. A formula is shown I in the description. The compound prepared by the synthesis method is a useful immediate or product synthesized by a plurality of medicines.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD
2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid
ActiveCN107383035BReasonable reaction process designThe method route is simpleOrganic chemistry methodsAcetic anhydrideLithium hydroxide
The invention relates to a preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid. The preparation method comprises 11 steps: carrying out esterification on a dicarboxylic acid piperidine compound 1 in thionyl chloride to obtain a diester compound 2; then reducing a pyridine ring by utilizing palladium carbon to obtain a compound 3; then taking the compound 3 to react with Boc acid anhydride to obtain a Boc protected compound 4; hydrolyzing the compound 4 by utilizing lithium hydroxide to obtain a dicarboxylic acid compound 5; carrying out ring closure in acetic anhydride to obtain a compound 6; carrying out reduction and ring opening by utilizing NaBH4 to obtain a mixture of position isomerism compounds 7A and 7B; carrying out the ring closure in the presence of iodomethane and potassium carbonate to generate position isomerism lactone 8A and 8B; step 8, reducing the 8A, obtained by column passing separation, by utilizing DIBAH (Diisobutylaluminum Hydride) to obtain a compound 9; carrying out Wittig reaction to obtain a compound 10; carrying out Michael addition under an alkaline condition to obtain a compound 11; finally, hydrolyzing the compound 11 under the alkaline condition to obtain a final compound.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD +3
Preparation method of 1-benzyl-8-tert-butyl-2-(hydroxymethyl)-diazaspirane decane dicarboxylate
InactiveCN107383007AHigh yieldThe reaction is easy to scale upOrganic chemistryGrignard reagentBenzyl chloroformate
The invention relates to a preparation method of 1-benzyl-8-tert-butyl-2-hydroxymethyl-1,8-diazaspirane[4.5]decane-1,8-dicarboxylate, mainly aiming at solving the technical problem that a suitable industrial synthesis method does not exist at present. The preparation method comprises six steps: firstly, taking a compound 1 and oxalyl chloride to react in dichloromethane, so as to generate a compound 2; then obtaining a compound 3 under the action of triethylamine; then taking the compound 3 to react with a vinyl Grignard reagent, so as to obtain a compound 4; then taking the compound 4 and a compound 5 to react under the condition that DBU is used as alkali, so as to obtain a compound 6; carrying out hydrogenation ring closure to obtain a compound 7; finally, taking the compound 7 to react with benzyl chloroformate under an alkaline condition, so as to obtain a final product 8. The compound prepared by the preparation method is used as a useful intermediate or product for synthesizing various medicines.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD +4
Preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid
ActiveCN107383035AReasonable reaction process designThe method route is simpleOrganic chemistry methodsAcetic anhydrideTert-Butyloxycarbonyl protecting group
The invention relates to a preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid. The preparation method comprises 11 steps: carrying out esterification on a dicarboxylic acid piperidine compound 1 in thionyl chloride to obtain a diester compound 2; then reducing a pyridine ring by utilizing palladium carbon to obtain a compound 3; then taking the compound 3 to react with Boc acid anhydride to obtain a Boc protected compound 4; hydrolyzing the compound 4 by utilizing lithium hydroxide to obtain a dicarboxylic acid compound 5; carrying out ring closure in acetic anhydride to obtain a compound 6; carrying out reduction and ring opening by utilizing NaBH4 to obtain a mixture of position isomerism compounds 7A and 7B; carrying out the ring closure in the presence of iodomethane and potassium carbonate to generate position isomerism lactone 8A and 8B; step 8, reducing the 8A, obtained by column passing separation, by utilizing DIBAH (Diisobutylaluminum Hydride) to obtain a compound 9; carrying out Wittig reaction to obtain a compound 10; carrying out Michael addition under an alkaline condition to obtain a compound 11; finally, hydrolyzing the compound 11 under the alkaline condition to obtain a final compound.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD +3
Preparation method of 1-phenylmethyl-5-(tert-butyloxycarbonyl) octahydropyrrolopyrrole-2-carboxylic acid
ActiveCN111620877AReasonable reaction process designMethod route shortOrganic chemistry methodsBulk chemical productionTert-Butyloxycarbonyl protecting groupEthylic acid
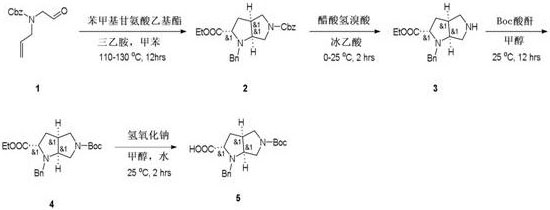
The invention relates to a preparation method of 1-phenylmethyl-5-(tert-butyloxycarbonyl) octahydropyrrolopyrrole-2-carboxylic acid, and mainly solves the technical problem that no suitable industrialsynthesis method exists at present. The method comprises the following four steps: 1, adding triethylamine into anhydrous toluene to obtain a compound 2 from a compound 1 and ethyl phenylmethylglycinate; 2, dissolving the compound 2 in glacial acetic acid under the action of hydrobromic acid acetate to obtain a compound 3; 3, dissolving the compound 3 in methanol, and adding Boc anhydride to obtain a compound 4; and 4, dissolving the compound 4 in water and methanol, adding sodium hydroxide, and hydrolyzing to obtain a compound 5, wherein the reaction formula is shown in the specification.
Owner:WUXI APPTEC
A kind of preparation method of (2,4,5,7-tetrahydropyrano[3,4-c]pyrazol-7-yl)methanol
ActiveCN110483534BShort synthetic routeHigh yieldOrganic chemistryMethane DichloridePyrazolylchalcone
The invention relates to a preparation method of (2,4,5,7-tetrahydropyrano[3,4-c]pyrazol-7-yl)methanol, which mainly solves the technical problem that there is no suitable industrial synthesis method at present. The present invention is divided into ten steps: first, compound 1 and triethyl orthoformate generate compound 2 under the action of boron trifluoride ether, then react with benzylhydrazine to obtain compound 3, and react with acetyl chloride to obtain compound 4, then obtain compound 4 in two Add N-bromosuccinimide to the solution of methyl chloride to obtain compound 5, react with ethylene boron trifluoride potassium salt to obtain compound 6, and obtain compound 7 with sodium hydroxide, and obtain compound 7 with N-bromosuccinimide Compound 8 was obtained by the reaction, compound 9 was obtained under the action of methanesulfonic acid, compound 10 was obtained by reacting with silver nitrate in acetonitrile solution, and product 11 was obtained by reacting with hydrogen gas under the catalyst palladium carbon. The compounds obtained in the present invention are useful intermediates or products of many pharmaceutical synthesis.
Owner:上海药明康德新药开发有限公司 +1
Preparation method of tertiary butyl 7-hydroxyhexahydrofuro-[3,2-b] pyridine-4(2H)-carboxylic ester
ActiveCN107188894AThe synthetic route is reliableThe reaction is easy to scale upOrganic chemistryDrugs synthesisPyridine
The invention relates to a preparation method of (3aR, 7S, 7aR)-tertiary butyl 7-hydroxyhexahydrofuro-[3,2-b] pyridine-4(2H)-carboxylic ester, and mainly solves the technical problem of no method suitable for industrial synthesis currently. The preparation method disclosed by the invention comprises nine steps in total; in the ninth step, under the action of sodium borohydride, a pair of compounds 12 and 12A are obtained. ( The structural formulas of the compounds are shown in the description) The compounds obtained by the preparation method are useful intermediates or products synthesized by various medicaments.
Owner:上海合全医药有限公司
A kind of synthetic method of compound ticagrelor and its synthetic intermediate
ActiveCN107513070BMild reaction conditionsSimple reaction conditionsOrganic chemistryTicagrelorSynthesis methods
The invention discloses a method for synthesizing a compound ticagrelor and a synthesized intermediate thereby. The method comprises the following steps: carrying out a substitution reaction between a compound (2) and a compound (3) to prepare a compound (4); carrying out a reduction reaction on the compound (4) to prepare a compound (5); carrying out a chlorination reaction on the compound (5) to prepare a compound (6); carrying out a substitution reaction between the compound (6) and a compound (7) to prepare a compound (8); and finally, carrying out a hydroxyl deprotection reaction on the compound (8), thereby obtaining the ticagrelor (1). The reaction formulas are as shown in the specification. The invention provides a novel method for synthesizing ticagrelor. The synthetic method has the advantages of novel technical route, simple and convenient operation, high synthesis yield, high product purity, cheap and readily available raw materials and the like, and is suitable for industrialized production. Meanwhile, the synthesized ticagrelor intermediate provides a novel intermediate raw material for preparation of the ticagrelor.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
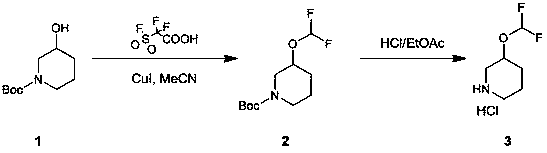
Preparation method of 3-(difluoromethoxy) piperidine hydrochloride
InactiveCN109627207AShort synthetic routeThe reaction is easy to scale upOrganic chemistrySynthesis methodsEthyl acetate
The invention relates to a preparation method of 3-(difluoromethoxy) piperidine hydrochloride. The main technical problem is solved that in the prior art, no suitable industrialized synthesis method exists. The preparation method comprises the two steps that in the first step, a compound 1 is added into acetonitrile, copper iodide is added into a solution, a mixture is heated, then 2-(fluorosulfonyl) difluoroacetic acid, and after a reaction is ended, a compound 2 is obtained through purification of after-treatment; in the second step, the compound 2 is stirred in an ethyl acetate hydrochloride solution to obtain a compound 3, wherein the reaction formula is shown in the specification. The obtained compound is an intermediate or a product which is synthesized by many medicines.
Owner:WUXI APPTEC (TIANJIN) CO LTD
Tert-butylhexahydro-2H-pyrrole[1,4]oxazepine-7(3H)-carboxylic ester preparation method
InactiveCN110845515AHigh yieldThe reaction is easy to scale upOrganic chemistryHydroxylamineEthylic acid
The invention relates to a tert-butylhexahydro-2H-pyrrole[1,4]oxazepine-7(3H)-carboxylic ester preparation method. A purpose of the invention is mainly to solve the technical problem of no industrialsynthesis method in the prior art. According to the invention, the method comprises the following six steps: generating a compound 2 from a compound 1 and N-benzyl hydroxylamine under the action of potassium acetate, 2, carrying out ring opening under the action of raney nickel to obtain a compound 3, carrying out a reaction on the compound 3 and chloroacetyl chloride to obtain a compound 4, obtaining a cyclization product compound 5 under the action of potassium tert-butoxide, carrying out borane reduction to obtain a compound 6, and finally obtaining a target product compound 7 under the action of palladium carbon. According to the invention, the compound obtained by the method is a useful intermediate or product for synthesizing a lot of medicines.
Owner:WUXI APPTEC
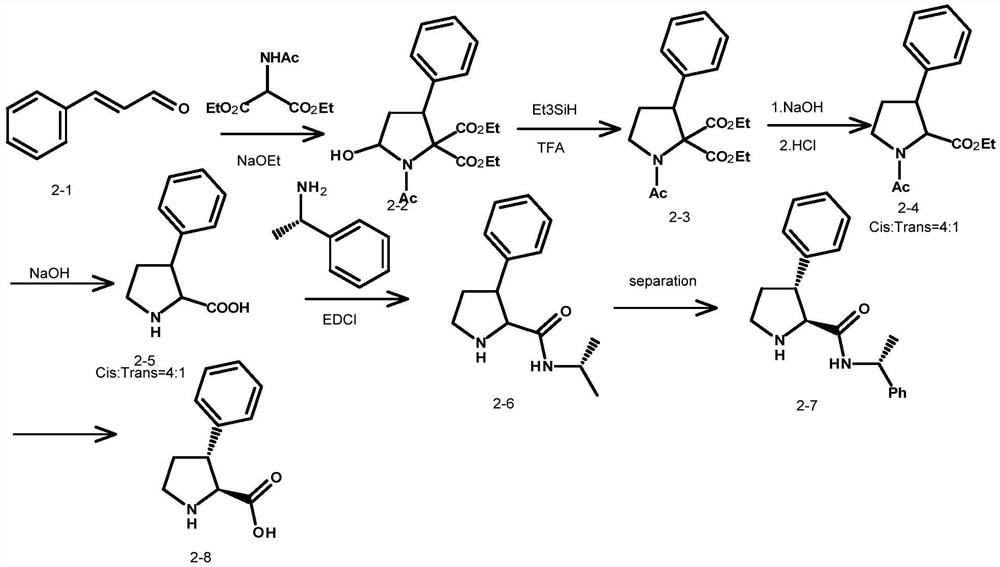
An intermediate for synthesizing (2s,3r)-3-substituted phenylpyrrolidine-2-carboxylic acid and its preparation method and application
ActiveCN110498808BThe synthetic route is reliableThe reaction is easy to scale upOrganic chemistry methodsBenzeneCombinatorial chemistry
The present invention relates to an intermediate of (2S,3R)-3-substituted phenylpyrrolidine-2-carboxylic acid and its preparation method and application, mainly solving the problem of (2S,3R)-3-substituted phenylpyrrolidine-2-carboxylic acid 2‑Carboxylic acids have no technical problems amenable to industrial synthetic methods. The present invention uses compound II and halogenated benzene as raw materials, and under the action of organometallic reagents, it is prepared through successive reaction steps such as Michael addition reaction, reduction reaction, debenzylation reaction, Boc protection reaction, oxidation and removal of Boc protection group, etc. Compound I ((2S,3R)-3-substituted phenylpyrrolidine-2-carboxylic acid) is obtained. The method is convenient to operate, stable in yield and suitable for large-scale production.
Owner:浙江晖石药业有限公司
Preparation method of tert-butyl 7-hydroxyhexahydrofuro[3,2-b]pyridine-4(2h)-carboxylate
ActiveCN107188894BThe synthetic route is reliableThe reaction is easy to scale upOrganic chemistryFuranDrugs synthesis
The invention relates to a preparation method of (3aR, 7S, 7aR)-tertiary butyl 7-hydroxyhexahydrofuro-[3,2-b] pyridine-4(2H)-carboxylic ester, and mainly solves the technical problem of no method suitable for industrial synthesis currently. The preparation method disclosed by the invention comprises nine steps in total; in the ninth step, under the action of sodium borohydride, a pair of compounds 12 and 12A are obtained. ( The structural formulas of the compounds are shown in the description) The compounds obtained by the preparation method are useful intermediates or products synthesized by various medicaments.
Owner:上海合全医药有限公司
A kind of synthetic method of 4-(tert-butoxycarbonyl) octahydrofuro[3,2-b]pyridine-6-carboxylic acid
ActiveCN105418620BReduce pollutionMethod route shortOrganic chemistryTert-Butyloxycarbonyl protecting groupAcetylation
The invention relates to a synthesis method of 4-(tert-butoxycarbonyl) octahydrofuro[3,2-b]pyridine-6-carboxylic acid, and mainly solves the technical problem that there is no suitable industrial synthesis method at present. The present invention is divided into six steps, firstly, compound 2 is obtained by esterification of compound 1, then compound 3 is obtained by reacting with iodine, compound 4 is obtained after acetylation of compound 3, compound 5 is obtained by coupling again, compound 6 is obtained by ring closure, and compound 6 is obtained by hydrogenation 7, and then reacted with Boc anhydride to obtain compound 8, which was hydrolyzed to obtain the final compound 9. The reaction formula is as follows: .
Owner:WUXI APPTEC (TIANJIN) CO LTD
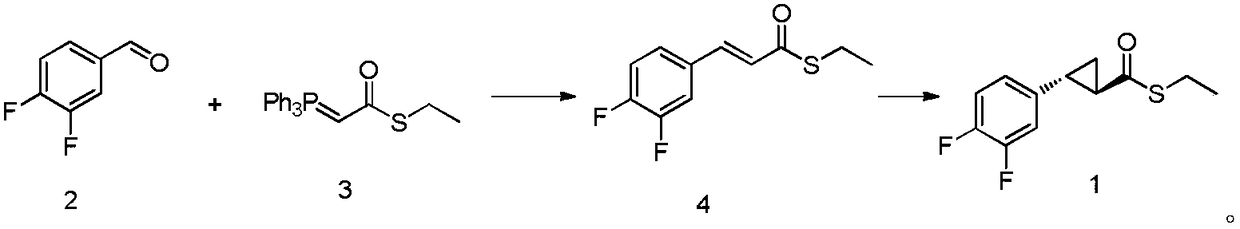
A kind of synthetic method of ticagrelor intermediate and its intermediate
ActiveCN107118141BNovel technical routeSimple and fast operationOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesTicagrelorSynthesis methods
The invention discloses a synthesis method of a ticagrelor intermediate. The method comprises the steps of allowing a compound (2) and a compound (3) to perform a coupling reaction to form a compound (4), and allowing the compound (4) to perform a rhodium-catalyzed asymmetric ternary cyclization reaction to form a ticagrelor intermediate compound (1), wherein a reaction formula of the method is shown in the description. The synthesis method has the advantages that the method is novel in technical route, easy and simple to operate, high in synthesis productivity and suitable for industrial production, a product is high in purity, and raw materials are cheap and easy to obtain. The compound (1) prepared by the rhodium-catalyzed asymmetric ternary cyclization reaction is a novel catalytical system; the novel system has the characteristics of mild reaction conditions, simple post-treatment, lower overall cost, relatively easy amplification of the reaction and the like; at the same time, the synthesized ticagrelor intermediate provides an intermediate raw material for preparation of ticagrelor and serves as an important intermediate for synthesis of ticagrelor.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
1-Benzyl-5-(tert-butoxycarbonyl)octahydropyrrolopyrrole-2-carboxylic acid preparation method
ActiveCN111620877BReasonable reaction process designMethod route shortOrganic chemistry methodsBulk chemical productionTert-Butyloxycarbonyl protecting groupEthylic acid
The invention relates to a method for preparing 1-benzyl-5-(tert-butoxycarbonyl) octahydropyrrolopyrrole-2-carboxylic acid, which mainly solves the technical problem that there is no suitable industrial synthesis method at present. The present invention is divided into four steps. In the first step, at first compound 2 is obtained by adding triethylamine into anhydrous toluene with compound 1 and benzylglycine ethyl ester. In the second step, compound 2 is dissolved in glacial acetic acid in acetic acid hydrobromic acid Compound 3 is obtained under the action of the third step. Compound 3 is dissolved in methanol, and then Boc anhydride is added to obtain compound 4. In the fourth step, compound 4 is dissolved in water and methanol, and sodium hydroxide is added to be hydrolyzed into compound 5. The reaction formula is as follows: .
Owner:WUXI APPTEC
Preparation method of 8-tert-butyl-1-ethyl-6,7-dihydro-5H-imidazo[1,5-a][1,4]diazepine-1,8(9H)-dicarboxylic acid ester
InactiveCN110563731AShort synthetic routeSimple responseOrganic chemistryEthyl phosphateDrugs synthesis
The invention relates to a preparation method of 8-tert-butyl-1-ethyl-6,7-dihydro-5H-imidazo[1,5-a][1,4]diazepine-1,8(9H)-dicarboxylic acid ester. A purpose of the present invention is to mainly solvethe technical problem that no suitable industrial synthesis method exists at present. According to the present invention, the method comprises nine steps, the synthetic route is defined in the specification, and the obtained compound can be used as the useful intermediate or product for synthesizing a plurality of medicines.
Owner:上海药明康德新药开发有限公司
A kind of preparation method of tert-butyl 1-hydroxyl-8-azaspirane[4,5]decane-8-carboxylate
Owner:WUXI APPTEC (TIANJIN) CO LTD
Ticagrelor intermediate and synthesis method thereof
ActiveCN107118141ANovel technical routeSimple and efficient operationOrganic-compounds/hydrides/coordination-complexes catalystsAsymmetric synthesesCombinatorial chemistryRh element
The invention discloses a synthesis method of a ticagrelor intermediate. The method comprises the steps of allowing a compound (2) and a compound (3) to perform a coupling reaction to form a compound (4), and allowing the compound (4) to perform a rhodium-catalyzed asymmetric ternary cyclization reaction to form a ticagrelor intermediate compound (1), wherein a reaction formula of the method is shown in the description. The synthesis method has the advantages that the method is novel in technical route, easy and simple to operate, high in synthesis productivity and suitable for industrial production, a product is high in purity, and raw materials are cheap and easy to obtain. The compound (1) prepared by the rhodium-catalyzed asymmetric ternary cyclization reaction is a novel catalytical system; the novel system has the characteristics of mild reaction conditions, simple post-treatment, lower overall cost, relatively easy amplification of the reaction and the like; at the same time, the synthesized ticagrelor intermediate provides an intermediate raw material for preparation of ticagrelor and serves as an important intermediate for synthesis of ticagrelor.
Owner:HUAIYIN INSTITUTE OF TECHNOLOGY
A kind of preparation method of 2-(1-(tert-butoxycarbonyl) azetidinyl-3-) cyclopropyl formic acid
ActiveCN105315188BLow priceAvoid flammable and explosive hazardsOrganic chemistryTert-Butyloxycarbonyl protecting groupSynthesis methods
The invention relates to a preparation method of 2-(1-(tertbutyloxycarbonyl)azacyclobutyl-3-yl)cyclopropanecarboxylic acid. The preparation method solves the problem that the existing appropriate 2-(1-(tertbutyloxycarbonyl)azacyclobutyl-3-yl)cyclopropanecarboxylic acid industrial synthesis method does not exist. The preparation method comprises that 1, a compound 1 and iodomethane undergo a reaction under alkaline conditions to produce a compound 2, 2, the compound 2 is treated by sodium borohydride to form a compound 3, 3, the compound 3 is oxidized by Dess-Martin periodinane to form a compound 4, 4, the compound 4 and a compound 5 undergo a reaction to produce a compound 6, 5, the compound 6 and trimethylsulfoxonium iodide undergo a reaction to produce a compound 7, and 6, the compound 7 is hydrolyzed under alkaline conditions to form an end product compound 8. The reaction equation is shown in the following description. The 2-(1-(tertbutyloxycarbonyl)azacyclobutyl-3-yl)cyclopropanecarboxylic acid is a useful intermediate or product for synthesis of many drugs.
Owner:武汉药明康德新药开发有限公司
Preparation method of tertiary butyl-1-methyl-5-oxysub unit triazaspiro[5.5] undecane-8-formyl ester
InactiveCN109485648AReduce the temperatureShort synthetic routeOrganic chemistryEthylenediamineReaction temperature
The invention relates to a preparation method of tertiary butyl-1-methyl-5-oxysub unit triazaspiro[5.5] undecane-8-formyl ester and primarily solves the technical problem that proper industrial synthetic methods are not available in the prior art. The preparation method comprises two steps: S1, adding ethanediamine and benzyl triethyl ammonium chloride by taking dichloromethane as a solvent, reducing the temperature of a reaction system to 5 DEG C and dropwise adding an aqueous solution of sodium hydroxide, stirring the mixture to react, dissolving N-Boc-3-piperidone and chloroform in dichloromethane into the reaction system when the temperature of the system is raised to 10 DEG C, and stirring the mixture to react when the reaction temperature is 15 DEG C to obtain a compound 3; and S2, dissolving the compound 3 in methanol at room temperature, adding formaldehyde and acetic acid, reducing the temperature of the reaction system to 0 DEG C, adding sodium cyanoborohydride, and carryingout a reaction at 20 DEG C and carrying out purification treatment to obtain a final product. The compound obtained by the invention provides an important intermediate for synthesizing many drugs.
Owner:WUXI APPTEC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Preparation method for bicyclo [1.1.1] pentane-1,3-dicarboxylic acid dimethylester Preparation method for bicyclo [1.1.1] pentane-1,3-dicarboxylic acid dimethylester](https://images-eureka.patsnap.com/patent_img/96ca2619-778e-460a-a141-f4fa7a99aceb/2014102411696100002DEST_PATH_IMAGE001.PNG)
![Preparation method for bicyclo [1.1.1] pentane-1,3-dicarboxylic acid dimethylester Preparation method for bicyclo [1.1.1] pentane-1,3-dicarboxylic acid dimethylester](https://images-eureka.patsnap.com/patent_img/96ca2619-778e-460a-a141-f4fa7a99aceb/DEST_PATH_IMAGE001.PNG)
![Synthesis method for 4-(tert-butoxycarbonyl) octahydrofuro[3,2-b] pyridine-6-carboxylic acid Synthesis method for 4-(tert-butoxycarbonyl) octahydrofuro[3,2-b] pyridine-6-carboxylic acid](https://images-eureka.patsnap.com/patent_img/c3c86bdd-fb12-4204-ac96-1283f3eeaca8/DEST_PATH_IMAGE002.PNG)
![Synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate Synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate](https://images-eureka.patsnap.com/patent_img/6d130abe-9731-4832-84f2-27c828084aaa/858410DEST_PATH_IMAGE002.png)
![Synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate Synthesis method of tert-butyl-8-oxo-2,6,9-triazaspiro[4.5]decane-2-formylate](https://images-eureka.patsnap.com/patent_img/6d130abe-9731-4832-84f2-27c828084aaa/DEST_PATH_IMAGE002.png)
![4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method 4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method](https://images-eureka.patsnap.com/patent_img/2399ecb6-2eb4-4916-b39d-5e924440f224/2014106610345100002DEST_PATH_IMAGE002.PNG)
![4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method 4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method](https://images-eureka.patsnap.com/patent_img/2399ecb6-2eb4-4916-b39d-5e924440f224/2014106610345100002DEST_PATH_IMAGE004.PNG)
![4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method 4-tert-butoxycarbonyl-hexahydro-2H-furo [3,2-b] pyrrole-6-carboxylic acid synthetic method](https://images-eureka.patsnap.com/patent_img/2399ecb6-2eb4-4916-b39d-5e924440f224/DEST_PATH_IMAGE002.PNG)
![6-tert-butyloxycarbonyl octahydro-2H-pyran[3,2-c]pyridine-8-carboxylic acid synthesis method 6-tert-butyloxycarbonyl octahydro-2H-pyran[3,2-c]pyridine-8-carboxylic acid synthesis method](https://images-eureka.patsnap.com/patent_img/da2a17e5-6bdd-4ee7-b53b-d70a621ade57/116401DEST_PATH_IMAGE001.PNG)
![6-tert-butyloxycarbonyl octahydro-2H-pyran[3,2-c]pyridine-8-carboxylic acid synthesis method 6-tert-butyloxycarbonyl octahydro-2H-pyran[3,2-c]pyridine-8-carboxylic acid synthesis method](https://images-eureka.patsnap.com/patent_img/da2a17e5-6bdd-4ee7-b53b-d70a621ade57/383175DEST_PATH_IMAGE002.PNG)
![Method for preparing 5-(tert-butoxycarbonyl)-2-oxooctahydropyrrolo[3, 4-b]pyrrole-3a-carboxylic acid Method for preparing 5-(tert-butoxycarbonyl)-2-oxooctahydropyrrolo[3, 4-b]pyrrole-3a-carboxylic acid](https://images-eureka.patsnap.com/patent_img/42fd5b46-df57-4fae-97ec-b587c98d7d43/DEST_PATH_IMAGE003.png)
![A preparation method of 4,5-dihydro-1h,3h-pyrrolo[1,2-a][1,4]diazepine-2,4-dicarboxylate-2-tert-butyl ester A preparation method of 4,5-dihydro-1h,3h-pyrrolo[1,2-a][1,4]diazepine-2,4-dicarboxylate-2-tert-butyl ester](https://images-eureka.patsnap.com/patent_img/01ef748e-1b6d-4cae-89b8-a99bf262626a/DEST_PATH_S.png)
![A preparation method of 4,5-dihydro-1h,3h-pyrrolo[1,2-a][1,4]diazepine-2,4-dicarboxylate-2-tert-butyl ester A preparation method of 4,5-dihydro-1h,3h-pyrrolo[1,2-a][1,4]diazepine-2,4-dicarboxylate-2-tert-butyl ester](https://images-eureka.patsnap.com/patent_img/01ef748e-1b6d-4cae-89b8-a99bf262626a/74978DEST_PATH_S.png)
![Preparation method of tert-butyl 2-iodomethyl-1-oxa-7-azaspirane [3,5] nonane-7-carboxylic ester Preparation method of tert-butyl 2-iodomethyl-1-oxa-7-azaspirane [3,5] nonane-7-carboxylic ester](https://images-eureka.patsnap.com/patent_img/10474158-e881-428a-9b6c-b41d7a7dd716/2015110068842100002DEST_PATH_IMAGE001.PNG)
![Preparation method of tert-butyl 2-iodomethyl-1-oxa-7-azaspirane [3,5] nonane-7-carboxylic ester Preparation method of tert-butyl 2-iodomethyl-1-oxa-7-azaspirane [3,5] nonane-7-carboxylic ester](https://images-eureka.patsnap.com/patent_img/10474158-e881-428a-9b6c-b41d7a7dd716/DEST_PATH_IMAGE002.PNG)
![Preparation method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid Preparation method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid](https://images-eureka.patsnap.com/patent_img/1cb2b678-4d12-49d1-8b23-6bfc618132d5/BSA0000140068260000011.png)
![Preparation method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid Preparation method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid](https://images-eureka.patsnap.com/patent_img/1cb2b678-4d12-49d1-8b23-6bfc618132d5/BSA0000140068260000021.png)
![Preparation method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid Preparation method of 2-tert-butyl-7-methyl-5-oxa-2,8-diazaspirane-[3,5]nonane-2,7-dicarboxylic acid](https://images-eureka.patsnap.com/patent_img/1cb2b678-4d12-49d1-8b23-6bfc618132d5/FSA0000140068250000011.png)
![2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid 2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid](https://images-eureka.patsnap.com/patent_img/2678f52b-b095-46d3-91e6-aa4bbbe3a737/DEST_PATH_IMAGE003.png)
![2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid 2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid](https://images-eureka.patsnap.com/patent_img/2678f52b-b095-46d3-91e6-aa4bbbe3a737/505010DEST_PATH_IMAGE004.png)
![2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid 2-((1s3ar7ar)-5-tert-butoxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid](https://images-eureka.patsnap.com/patent_img/2678f52b-b095-46d3-91e6-aa4bbbe3a737/600639DEST_PATH_IMAGE002.png)
![Preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid Preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid](https://images-eureka.patsnap.com/patent_img/3100eb7b-3fd2-4cc9-8776-f6c91c9defda/505010DEST_PATH_IMAGE004.png)
![Preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid Preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid](https://images-eureka.patsnap.com/patent_img/3100eb7b-3fd2-4cc9-8776-f6c91c9defda/600639DEST_PATH_IMAGE002.png)
![Preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid Preparation method of 2-((1S3aR7aR)-5-tert-butyloxycarbonyltetrahydrofuro[3,4]piperidine-1)acetic acid](https://images-eureka.patsnap.com/patent_img/3100eb7b-3fd2-4cc9-8776-f6c91c9defda/DEST_PATH_IMAGE003.png)
![Preparation method of tertiary butyl 7-hydroxyhexahydrofuro-[3,2-b] pyridine-4(2H)-carboxylic ester Preparation method of tertiary butyl 7-hydroxyhexahydrofuro-[3,2-b] pyridine-4(2H)-carboxylic ester](https://images-eureka.patsnap.com/patent_img/4909d73a-7ce7-40d5-a03e-dc6c936ab597/DEST_PATH_IMAGE004.png)
![Tert-butylhexahydro-2H-pyrrole[1,4]oxazepine-7(3H)-carboxylic ester preparation method Tert-butylhexahydro-2H-pyrrole[1,4]oxazepine-7(3H)-carboxylic ester preparation method](https://images-eureka.patsnap.com/patent_img/60c657f9-4c5c-49c3-8c95-f71b5560985f/510052DEST_PATH_IMAGE002.png)
![Preparation method of tert-butyl 7-hydroxyhexahydrofuro[3,2-b]pyridine-4(2h)-carboxylate Preparation method of tert-butyl 7-hydroxyhexahydrofuro[3,2-b]pyridine-4(2h)-carboxylate](https://images-eureka.patsnap.com/patent_img/7dc7db12-88e8-432f-97e4-2735b04653bd/DEST_PATH_IMAGE002.png)
![Preparation method of tert-butyl 7-hydroxyhexahydrofuro[3,2-b]pyridine-4(2h)-carboxylate Preparation method of tert-butyl 7-hydroxyhexahydrofuro[3,2-b]pyridine-4(2h)-carboxylate](https://images-eureka.patsnap.com/patent_img/7dc7db12-88e8-432f-97e4-2735b04653bd/DEST_PATH_IMAGE004.png)
![Preparation method of 8-tert-butyl-1-ethyl-6,7-dihydro-5H-imidazo[1,5-a][1,4]diazepine-1,8(9H)-dicarboxylic acid ester Preparation method of 8-tert-butyl-1-ethyl-6,7-dihydro-5H-imidazo[1,5-a][1,4]diazepine-1,8(9H)-dicarboxylic acid ester](https://images-eureka.patsnap.com/patent_img/9afa014a-97b9-44a9-8470-25c2f8c0aa58/2.png)
![Preparation method of 8-tert-butyl-1-ethyl-6,7-dihydro-5H-imidazo[1,5-a][1,4]diazepine-1,8(9H)-dicarboxylic acid ester Preparation method of 8-tert-butyl-1-ethyl-6,7-dihydro-5H-imidazo[1,5-a][1,4]diazepine-1,8(9H)-dicarboxylic acid ester](https://images-eureka.patsnap.com/patent_img/9afa014a-97b9-44a9-8470-25c2f8c0aa58/100002_1.png)
![A kind of preparation method of tert-butyl 1-hydroxyl-8-azaspirane[4,5]decane-8-carboxylate A kind of preparation method of tert-butyl 1-hydroxyl-8-azaspirane[4,5]decane-8-carboxylate](https://images-eureka.patsnap.com/patent_img/a92bdf18-00f5-4778-8135-6105748d2f4f/DEST_PATH_IMAGE003.png)
![Preparation method of tertiary butyl-1-methyl-5-oxysub unit triazaspiro[5.5] undecane-8-formyl ester Preparation method of tertiary butyl-1-methyl-5-oxysub unit triazaspiro[5.5] undecane-8-formyl ester](https://images-eureka.patsnap.com/patent_img/c7044b0b-67f3-4aab-88a3-94195af59246/292015DEST_PATH_IMAGE001.png)
![Preparation method of tertiary butyl-1-methyl-5-oxysub unit triazaspiro[5.5] undecane-8-formyl ester Preparation method of tertiary butyl-1-methyl-5-oxysub unit triazaspiro[5.5] undecane-8-formyl ester](https://images-eureka.patsnap.com/patent_img/c7044b0b-67f3-4aab-88a3-94195af59246/DEST_PATH_IMAGE001.png)