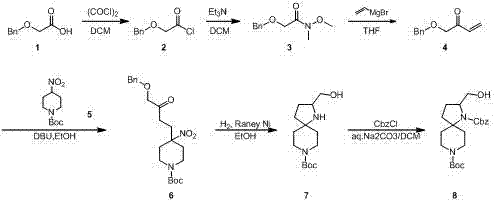
Preparation method of 1-benzyl-8-tert-butyl-2-(hydroxymethyl)-diazaspirane decane dicarboxylate
A technology of azaspirone and dicarboxylate, which is applied in the field of preparation of 1-benzyl 8-tert-butyl 2--diazaspiroane decane dicarboxylate, can solve the problem that there is no suitable industrial synthesis method and other problems, to achieve the effect of short synthetic route, easy reaction and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0010] Example 1: a. Compound 1 (3 g, 18 mmol) was dissolved in anhydrous dichloromethane (30 mL), the reaction solution was lowered to 0°C, oxalyl chloride (3 mL) was added dropwise thereto, and then N , N-dimethylformamide (0.03 mL), after the dropwise addition, the reaction was carried out at room temperature for 5 hours. The mixture was distilled under reduced pressure to obtain crude compound 2 (3 g) as a brown oil, with a yield of 100%.
[0011] b. Dissolve compound 2 (3 g, 18 mmol) and N,O-dimethylhydroxylamine hydrochloride (2.3 g, 23 mmol) in dichloromethane (40 mL), and cool the reaction system to 0°C, Therein, triethylamine (4.5 g, 45 mmol) was slowly added dropwise, and the reaction was controlled at zero to continue the reaction for 4 hours. TLC (petroleum ether / ethyl acetate volume ratio=3 / 1) showed that the reaction was complete. The reaction solution was washed twice with water and saturated brine respectively, dried over sodium sulfate, filtered and concentr...
Embodiment 2
[0016] Example 2: a. Compound 1 (300 g, 1.8 mol) was dissolved in anhydrous dichloromethane (2 L), the reaction solution was lowered to 0°C, oxalyl chloride (300 mL) was added dropwise thereto, and then N,N-Dimethylformamide (3 mL), after the dropwise addition, the reaction was carried out at room temperature for 5 hours. The mixture was distilled under reduced pressure to obtain compound 2 (332 g) as a brown oil, with a yield of 100%.
[0017] b. Dissolve compound 2 (332 g, 1.8 mol) and N,O-dimethylhydroxylamine hydrochloride (228 g, 2.3 mol) in dichloromethane (4 L), cool the reaction system to 0°C, Therein, triethylamine (454.5 g, 4.5 mol) was slowly added dropwise, and the reaction was controlled at zero to continue the reaction for 4 hours. TLC (petroleum ether / ethyl acetate volume ratio=3 / 1) showed that the reaction was complete. The reaction solution was washed twice with water and saturated brine respectively, dried over sodium sulfate, filtered and concentrated by d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com