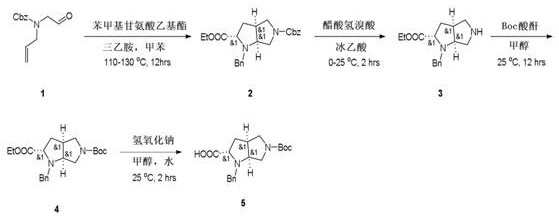
1-Benzyl-5-(tert-butoxycarbonyl)octahydropyrrolopyrrole-2-carboxylic acid preparation method
A technology of tert-butoxycarbonyl and octahydropyrrole, which is applied in the fields of organic chemistry, organic chemistry, bulk chemical production, etc., can solve the problems of no suitable industrial synthesis methods, and achieve reasonable reaction process design, convenient operation, and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0012] Step 1: Compound 1 (259 g 1.11 mol), ethyl benzylglycinate (251 g, 1.3 mol) and triethylamine (168 g, 1.7 mol) are dissolved in toluene (3.0 L), removed with a water separator, reflux at 110-130 °C for 12 hours, TLC (petroleum ether / ethyl acetate volume ratio = 5 / 1) shows that the raw material is completely consumed. Wash the mixture with 1 N HCl (600 mL x 2) and brine (600 mL), dry with anhydrous sodium sulfate, and concentrate to give orange oily compound 2 (380 g, yield: 100%).
[0013] Steps 2 and 3: Dissolve Compound 2 (101 g 0.25 mol) in glacial acetic acid (100 mL) and drop hydrobromic acid acetate (200 mL) at 0 °C. The mixture was stirred at room temperature at 25 ° C for 2 hours. TLC (Petroleum Ether / Ethyl Acetate = 4 / 1) indicates that the raw material is completely consumed. Concentrate the reaction solution to dryness and adjust the pH = 8-9 with an aqueous sodium carbonate solution. Then add methanol (200 ml), Boc anhydride (70 g, 0.4 mol). The resulting solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com