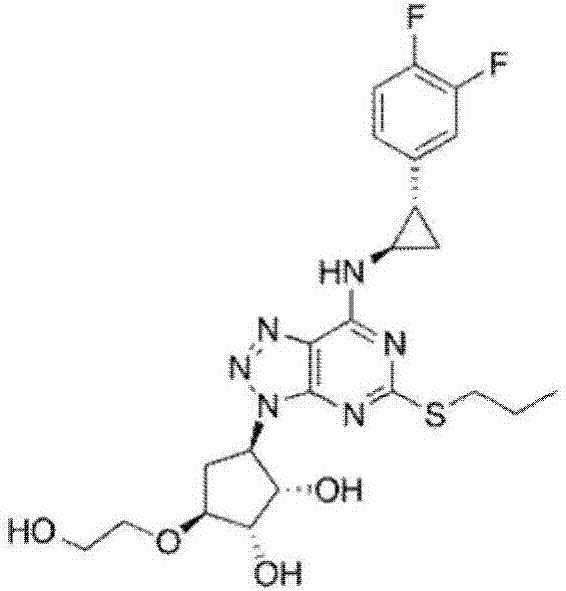
Ticagrelor intermediate and synthesis method thereof
A technology of ticagrelor and a synthesis method, applied in the field of drug synthesis, can solve the problems of being unable to be suitable for industrialized large-scale production, low product yield and high cost, and achieve the effects of novel technical route, good product purity and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
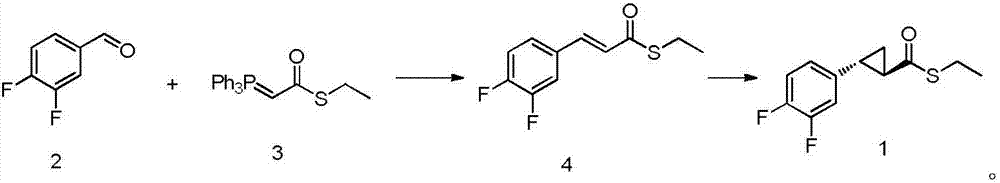
[0034] Preparation of compound 4:
[0035] Add 142g (1mol) of compound (2) and 382g (1.05mol) of compound (3) into 1L of dichloromethane into a 2L four-neck round bottom flask, stir at room temperature for 16 hours, filter, concentrate, and recrystallize the crude product from toluene The obtained intermediate compound (4) 226g. The mass yield of compound (4) was 159%, and the purity by HPLC was 99.02%.
[0036] 1 H NMR (500MHz, DMSO-d 6 )δ7.69(d, J=30.4Hz, 1H), 7.48(m, 1H), 7.11(m, 1H), 6.93(m, 1H), 6.81(d, J=30.1Hz, 1H), 3.15( q,J=13.1Hz,2H),1.31(t,J=13.2Hz,3H).
[0037] Preparation of intermediate compound 1:
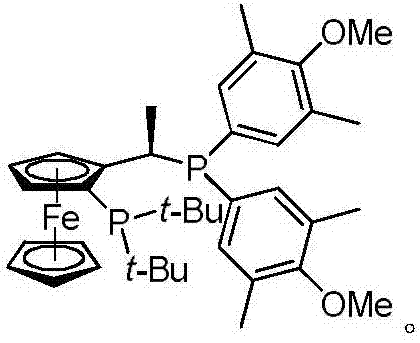
[0038] Add reaction solvent anhydrous dichloromethane 500mL and 220g (0.96mol) compound (4) in the four-neck round bottom flask of 2L, add catalyst again to be 5.5g (9.6mmol) Rh 2 (OCOt-Bu) 4 and ligand 5.6g (9.6mmol), after the addition, the reaction solution was cooled to -30°C, and then a toluene solution of diazomethane (500mL, 2M dichloromethane solution,...
Embodiment 2
[0043] According to the synthetic method of embodiment 1, the molar ratio of compound (2) and compound (3) compound 3 is 1:1.1, and the reaction solvent of compound (1) in the preparation is replaced with anhydrous ether, and the rhodium catalyst is RhCl 3 , the molar ratio of rhodium catalyst to compound (4) is 1:20, the molar ratio of ligand to rhodium catalyst is 1:1.2, and the reaction temperature is -80°C. Finally, the refined product of compound (1) was obtained, with a mass yield of 98.5%, and a purity of 99.55% by HPLC.
Embodiment 3
[0045] According to the synthetic method of embodiment 1, the molar ratio of compound (2) and compound (3) compound 3 is 1:1.2, and the reaction solvent of compound (1) in the preparation is replaced with anhydrous toluene, and the rhodium catalyst is Rh 2 (pfb) 4 , the molar ratio of rhodium catalyst to compound (4) is 1:80, the molar ratio of ligand to rhodium catalyst is 1:1.5, and the reaction temperature is 20°C. Finally, the refined product of compound (1) was obtained, with a mass yield of 98%, and a purity of 99.43% by HPLC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com