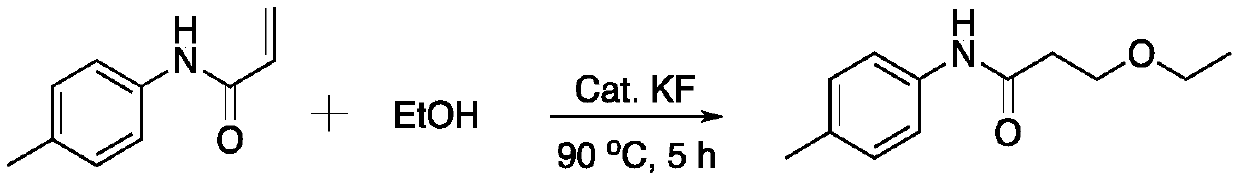
Synthesis method of 3-ethoxy-N-p-tolyl propionamide
A technology of tolylpropionamide and tolylacrylamide, which is applied in the field of synthesis of 3-ethoxy-N-p-tolylpropionamide, can solve problems such as difficult separation and recovery, pollution of the atmospheric environment, and large environmental impact, and achieve product Good purity, high selectivity, and easy recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
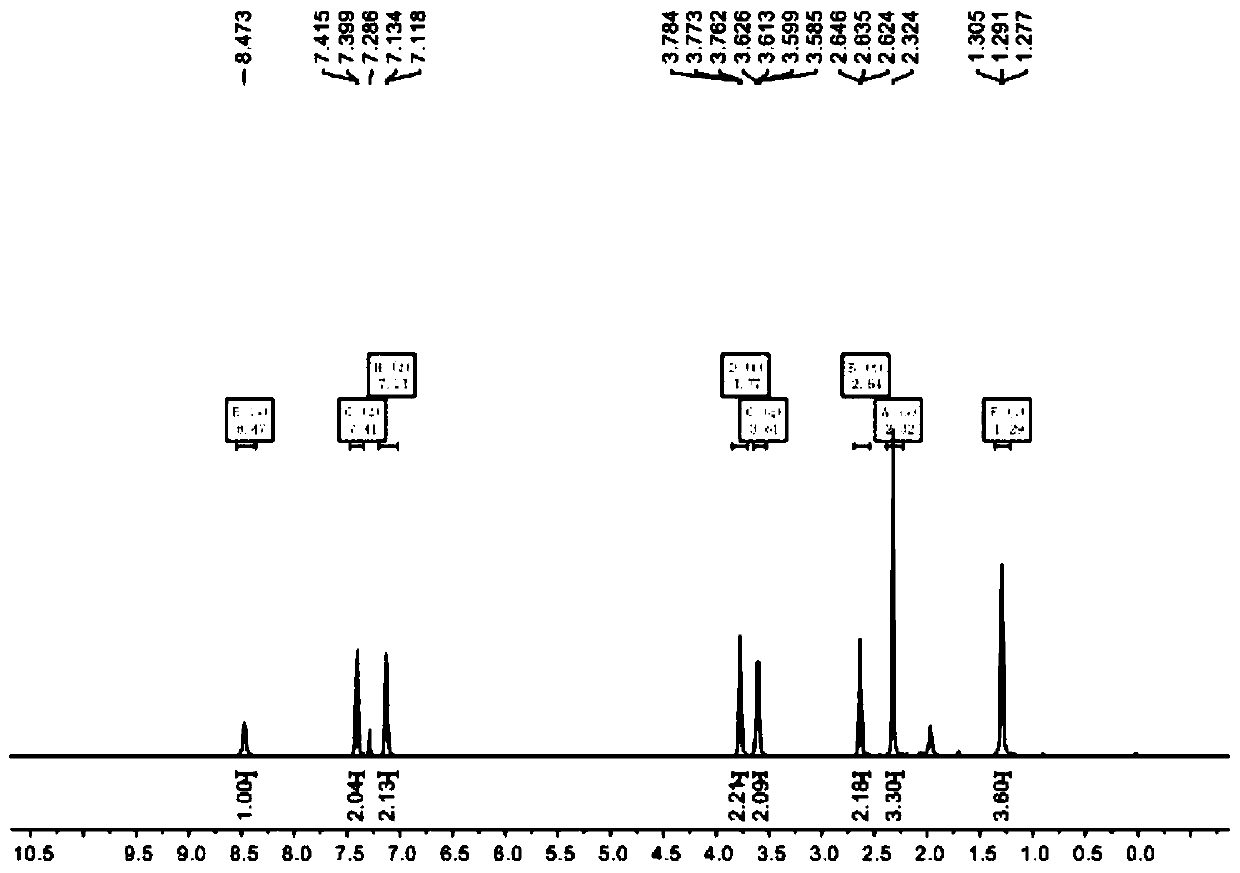
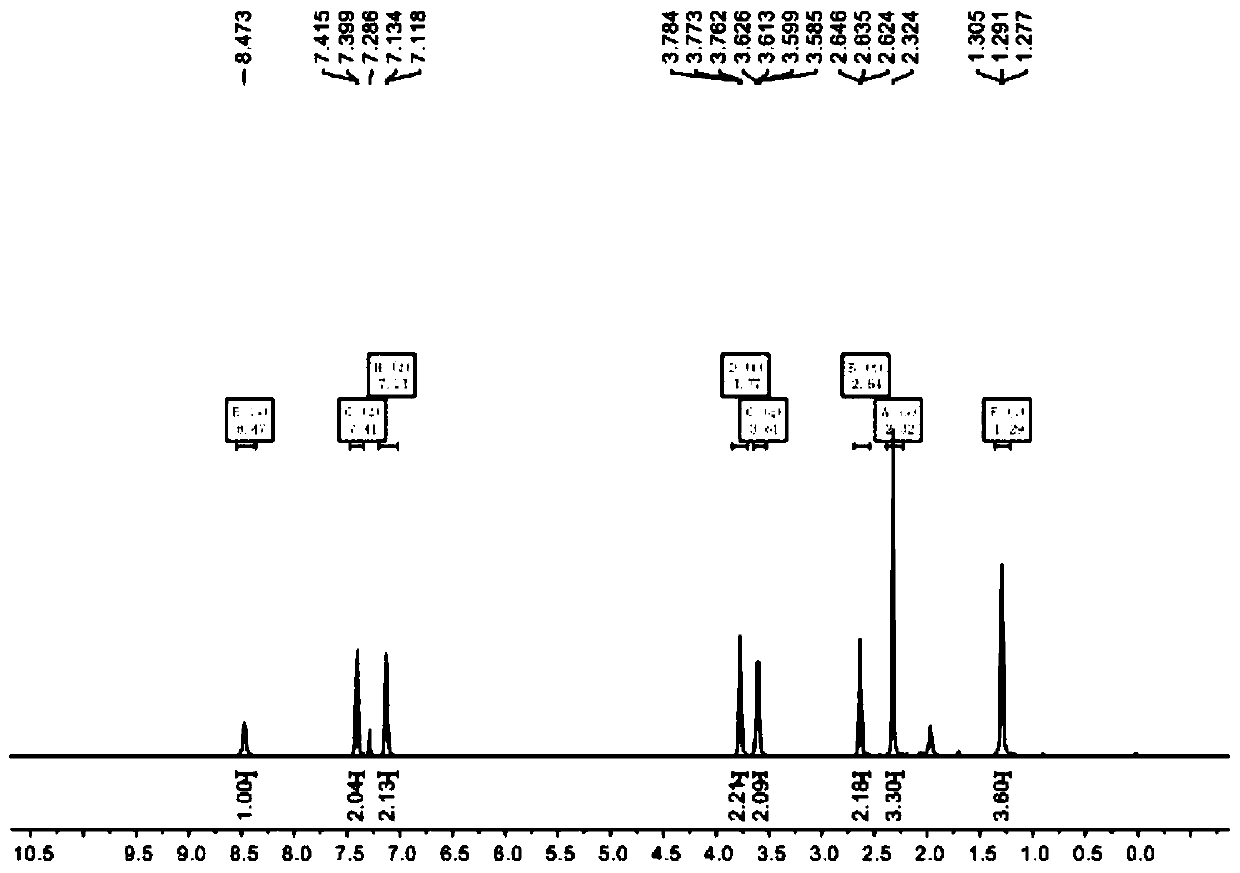
[0021] Add catalyst BaO (0.015g), N-p-tolylacrylamide (0.1mmol, 0.0161g), potassium fluoride (0.3mmol, 0.017g) and 3mL of ethanol into a 10mL reaction tube in sequence, and the reaction temperature is controlled at 90 ℃, and stirred for 5h. After the reaction is over, cool to room temperature, centrifuge the reaction system, take the supernatant liquid, and distill under reduced pressure (normal conditions: temperature 40°C, vacuum degree: -0.1MPa) to obtain 3-ethoxy-N -P-tolyl propionamide.
[0022] 1 HNMR(500MHz, CDCl 3 )δ8.47(s,1H),7.41(d,J=8.0Hz,2H), 7.13(d,J=7.9Hz,2H), 3.77(t,J=5.6Hz,2H), 3.61(q, J = 6.9Hz, 2H), 2.64 (t, J = 5.5Hz, 2H), 2.32 (s, 3H), 1.29 (t, J = 7.0Hz, 4H).
[0023] The equation involved in the reaction is as follows:
[0024]
specific Embodiment 2
[0026] Add the catalyst La to the 10mL reaction tube sequentially 2 O 3 (0.015g), N-p-tolylacrylamide (0.1mmol, 0.0161g), potassium fluoride (0.3mmol, 0.017g) and 3mL of ethanol, the reaction temperature was controlled at 90°C, and the reaction was stirred for 5h. After the reaction is finished, cool to room temperature, centrifuge the reaction system, take the supernatant liquid, and distill under reduced pressure to obtain 3-ethoxy-N-p-tolylpropionamide.
[0027] The equation involved in the reaction is as follows:
[0028]
specific Embodiment 3
[0030] Add the catalyst CeO sequentially to the 10mL reaction tube 2 (0.015g), N-p-tolylacrylamide (0.1mmol, 0.0161g), potassium fluoride (0.3mmol, 0.017g) and 3mL of ethanol, the reaction temperature was controlled at 90°C, and the reaction was stirred for 5h. After the reaction is completed, cool to room temperature, centrifuge the reaction system, take the supernatant liquid, and distill under reduced pressure to obtain 3-ethoxy-N-p-tolylpropionamide.
[0031] The equation involved in the reaction is as follows:
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com