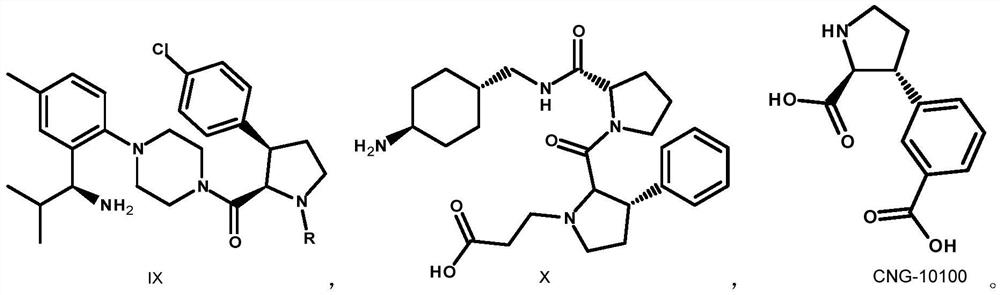
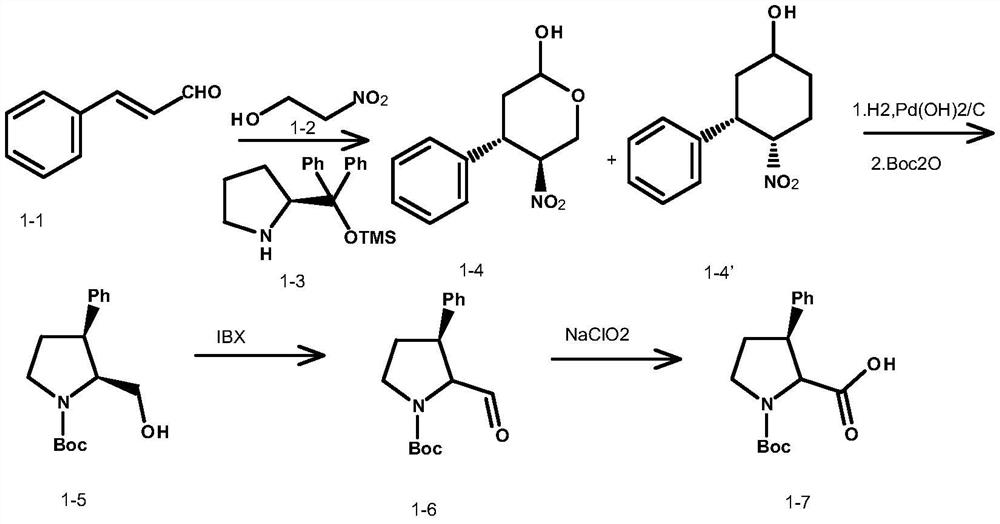
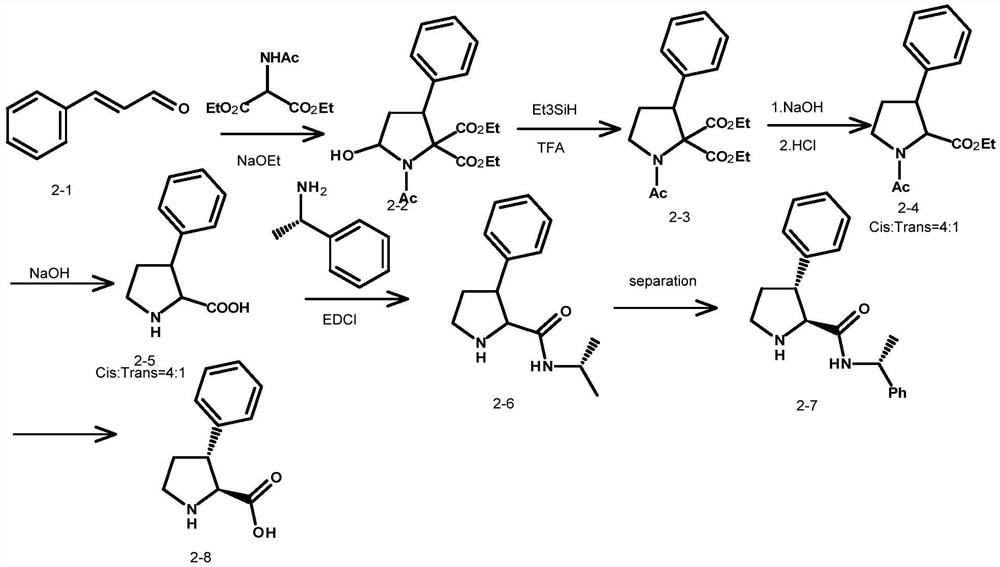
An intermediate for synthesizing (2s,3r)-3-substituted phenylpyrrolidine-2-carboxylic acid and its preparation method and application
A technology of trimethylchlorosilane and oxygen group, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of low total yield, poor stereoselectivity, long route and the like, and achieves the effects of convenient operation, easy reaction and reliable synthesis route.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055]
[0056] Preparation of Compound IV-1:
[0057]Bromobenzene (327.7g, 2.09mol, 3.0eq.) was dissolved in THF (500mL), cooled to -78°C under nitrogen protection, and n-BuLi (2.5M, 807mL, 2.9eq.) was added dropwise, and the addition was completed , keep stirring for 30min, add CuCN (97.68g, 1.04mol, 1.5eq.), raise the temperature to about -50°C, keep the reaction for 30min, below -78°C, dropwise add compound II (140.1g, 695.7mmol, 1eq.) THF (500mL) solution, after the addition was completed, TMSCl (188.9g, 1.74mol, 2.5eq.) was added dropwise, after the addition was completed, stirred for 30min, the temperature was naturally raised to -40°C, and the reaction was detected by TLC. At -40~0°C, add saturated ammonium chloride aqueous solution (2.0L) dropwise to quench the reaction, add EA (2.0L), extract and separate the liquid, and use saturated ammonium chloride aqueous solution (1.0L) and saturated chloride Wash with aqueous sodium solution (1.0L), dry over anhydrous magn...
Embodiment 2
[0069]
[0070] Preparation of Compound IV-2:
[0071] Compound III-2 (757.65g, 3.475mol, 5.0eq.) was dissolved in anhydrous THF (700mL), under nitrogen protection, Mg powder (83.4g, 3.475mol, 5.0eq.) was added, and a small amount of iodine was added to initiate the reaction at room temperature. After the reaction, heat to reflux and stir for 20 minutes, then add the suspension to CuBr-Me at -40°C 2 S (357.2g, 1.737mol, 2.5eq.) in 300mL diethyl ether solution, after stirring for 1h, the Grignard reagent was formed for later use. Compound II (140.1g, 695.75mmol, 1eq.) was dissolved in THF (500mL), cooled to -78°C, and CuCN (97.68g, 1.04mol, 1.5eq.) was added under nitrogen protection,
[0072] TMSCl (377.5g, 3.475mol, 5.0eq.) was incubated and reacted for 30min, below -78°C, added the spare Grignard reagent dropwise, after the addition was complete, stirred for 2h, and the temperature was naturally raised to -40°C, and the reaction was detected by TLC. At -40~0°C, add satu...
Embodiment 3
[0084]
[0085] Preparation of compound IV-3:
[0086] Compound III-3 (751.43g, 2.783mol, 4.0eq.) was dissolved in THF (800mL), cooled to -78°C under nitrogen protection, and n-BuLi (2.5M, 834mL, 3.0eq.) was added dropwise, After the feeding is completed, keep stirring for 30 minutes, then add CuBr-Me 2 S (213.8g, 1.04mol, 1.5eq.), the temperature was raised to about -50°C, and the reaction was incubated for 30min. Below -78°C, a THF (500mL) solution of compound II (140.1g, 695.75mmol, 1eq.) was added dropwise, After the addition was completed, TMSCl (264.6 g, 2.43 mol, 3.5 eq.) was added dropwise. After the addition was completed, the mixture was stirred for 30 minutes, and the temperature was naturally raised to -40°C. TLC detected that the reaction was complete. At -40~0°C, add saturated ammonium chloride aqueous solution dropwise to quench the reaction, add EA, extract and separate the liquids, wash the organic phase with saturated ammonium chloride aqueous solution an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| enantiomeric excess | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com