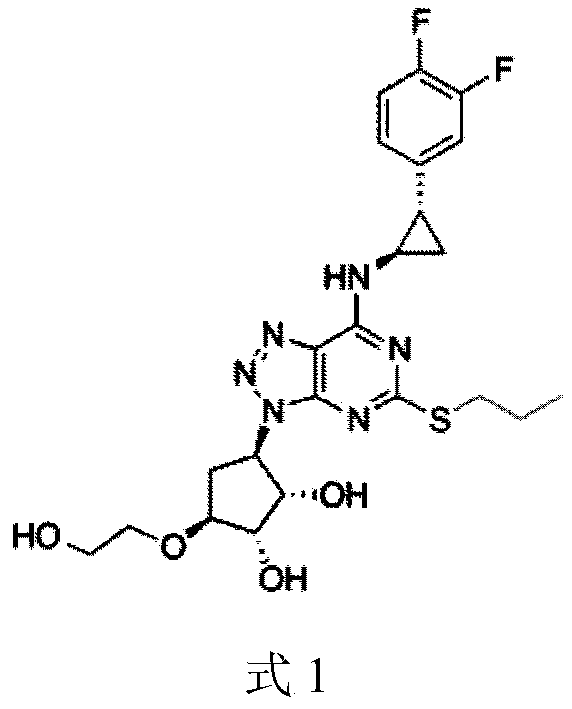
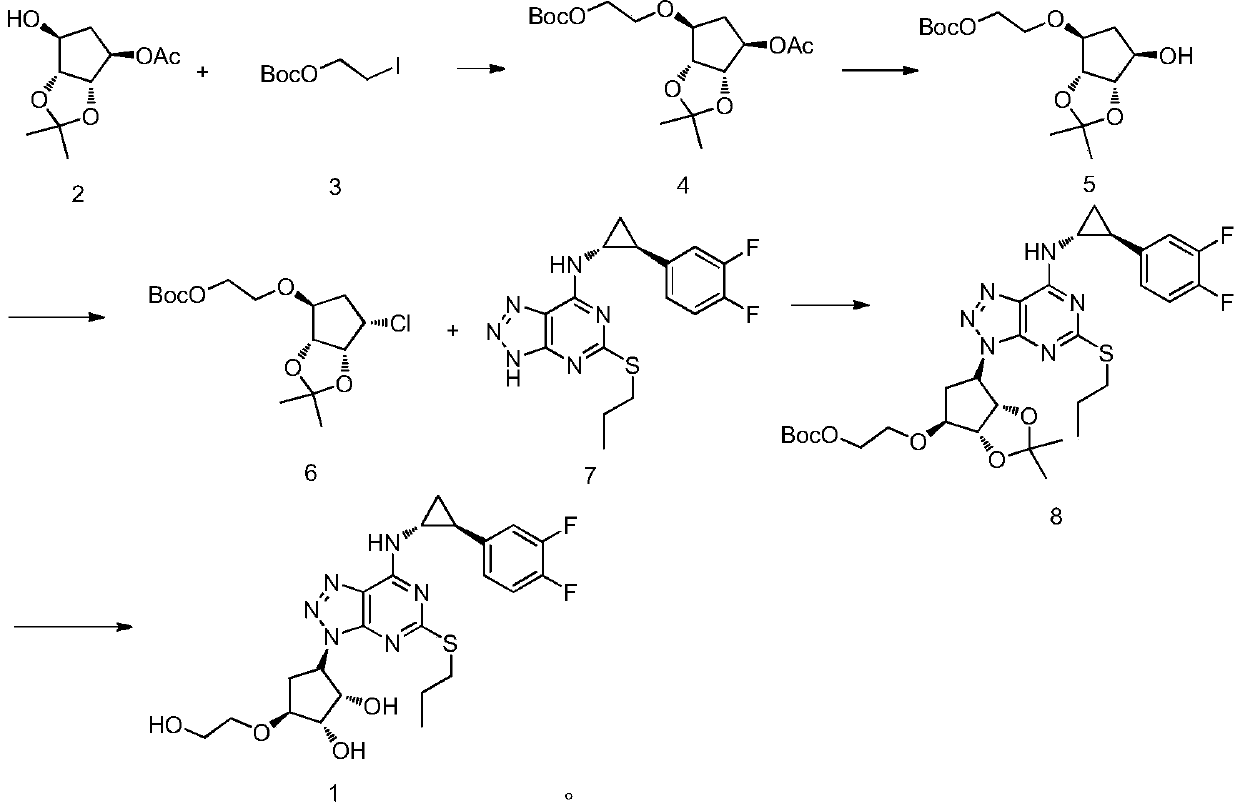
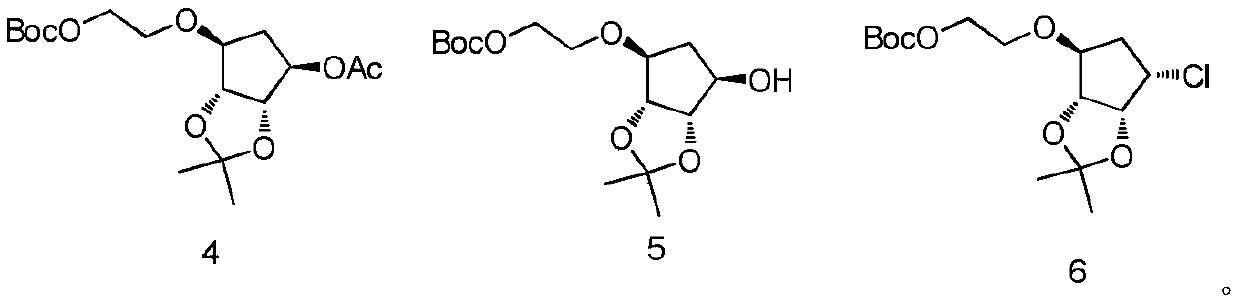
A kind of synthetic method of compound ticagrelor and its synthetic intermediate
A technology of ticagrelor and a synthesis method, applied in the field of drug synthesis, can solve problems such as being unable to be suitable for industrialized large-scale production, low product yield, poor quality and the like, and achieve the effects of novel technical route, good product purity and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of compound 4:
[0034] Under nitrogen protection, under the condition of 0 ℃, add 216g (1.0mol) compound (2) and 123g (1.1mol) potassium tert-butoxide in 2L anhydrous THF in 5L four-neck round bottom flask, control temperature If it exceeds 5°C, stir for 1 hour after the addition, then slowly add 286g of compound (3), raise the temperature to about 40°C, monitor the reaction by TLC, the reaction ends after 5 hours, cool down to room temperature, slowly add 2L of water, separate liquid, water phase Extract with ethyl acetate three times and add 1 L each time, combine the organic phases, and concentrate under reduced pressure to obtain the crude intermediate compound (4), which is recrystallized from ethyl acetate / n-heptane to obtain 313.2 g of the refined product.
[0035] The mass yield is 145%, and the HPLC detection purity: 99.11%.
[0036] 1 H NMR (500MHz, DMSO-d 6 )δ5.01(m,1H),4.43–4.26(m,3H),4.26–3.98(m,2H),3.65(t,J=14.6Hz,2H),2.19(m,1H),2.02(s ,3H...
Embodiment 2
[0058] According to the synthesis method of Example 1, the alkaline reagent in the preparation of compound 5 was replaced by potassium hydroxide (5% potassium hydroxide aqueous solution), the reaction solvent was replaced by dichloromethane, and the reaction temperature was -80°C.
[0059] The mass yield is 90.5%, and the HPLC detection purity: 99.83%.
[0060] In the preparation of compound 6, the chlorination reagent was replaced by SOCl 2 , the reaction solvent was replaced by anhydrous DMSO, and the reaction temperature was 10°C.
[0061] The mass yield is 102%, and the HPLC detection purity: 99.15%.
[0062] In the preparation of compound 8, the base was replaced by sodium tert-butoxide, the reaction solvent was replaced by 2-methyltetrahydrofuran, and the reaction temperature was 45°C.
[0063] The mass yield is 188.59%, and the HPLC detection purity is 99.23%.
[0064] In the preparation of compound 1 ticagrelor, the acid was replaced with hydrogen chloride methanol ...
Embodiment 3
[0066] According to the synthesis method of Example 1, the alkaline reagent in the preparation of compound 5 was replaced by sodium bicarbonate (10% aqueous sodium bicarbonate solution by mass fraction), the reaction solvent was replaced by tert-butanol, and the reaction temperature was 20°C.
[0067] The mass yield is 91.3%, and the HPLC detection purity: 99.78%.
[0068] In the preparation of compound 6, the chlorination reagent was replaced by PCl 5 , the reaction solvent was replaced by anhydrous TBME, and the reaction temperature was 0°C.
[0069] The mass yield is 105%, and the HPLC detection purity: 99.55%.
[0070] In the preparation of compound 8, the base was replaced by KHMDS, the reaction solvent was replaced by xylene, and the reaction temperature was 0°C.
[0071] The mass yield is 188.35%, and the HPLC detection purity: 99.01%.
[0072] In the preparation of compound 1 ticagrelor, the acid was replaced with hydrogen chloride methanol solution, and the mass yi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com