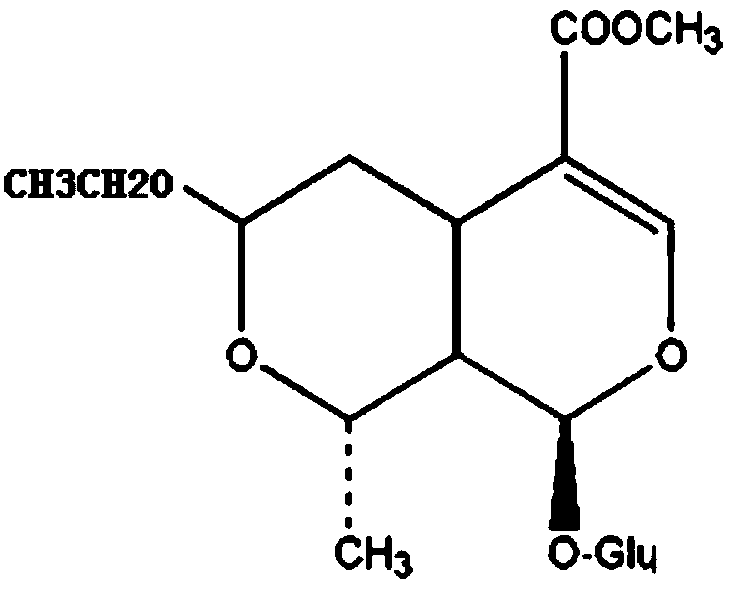
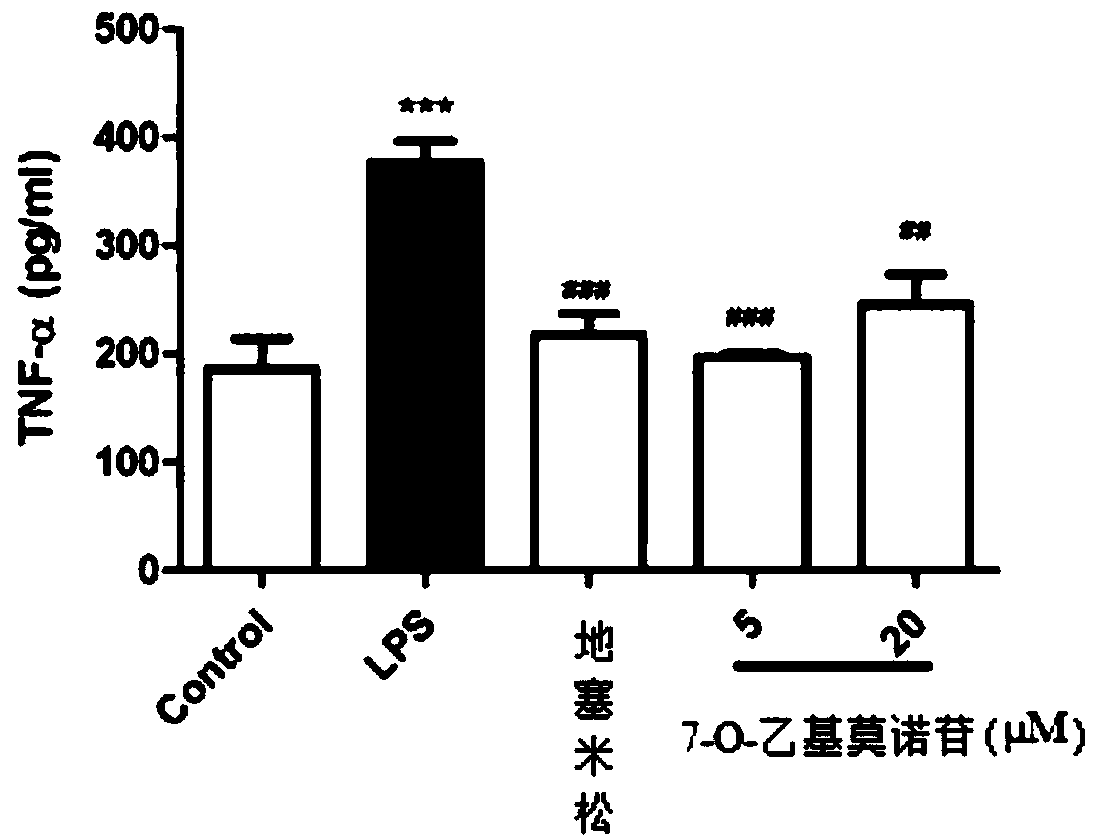
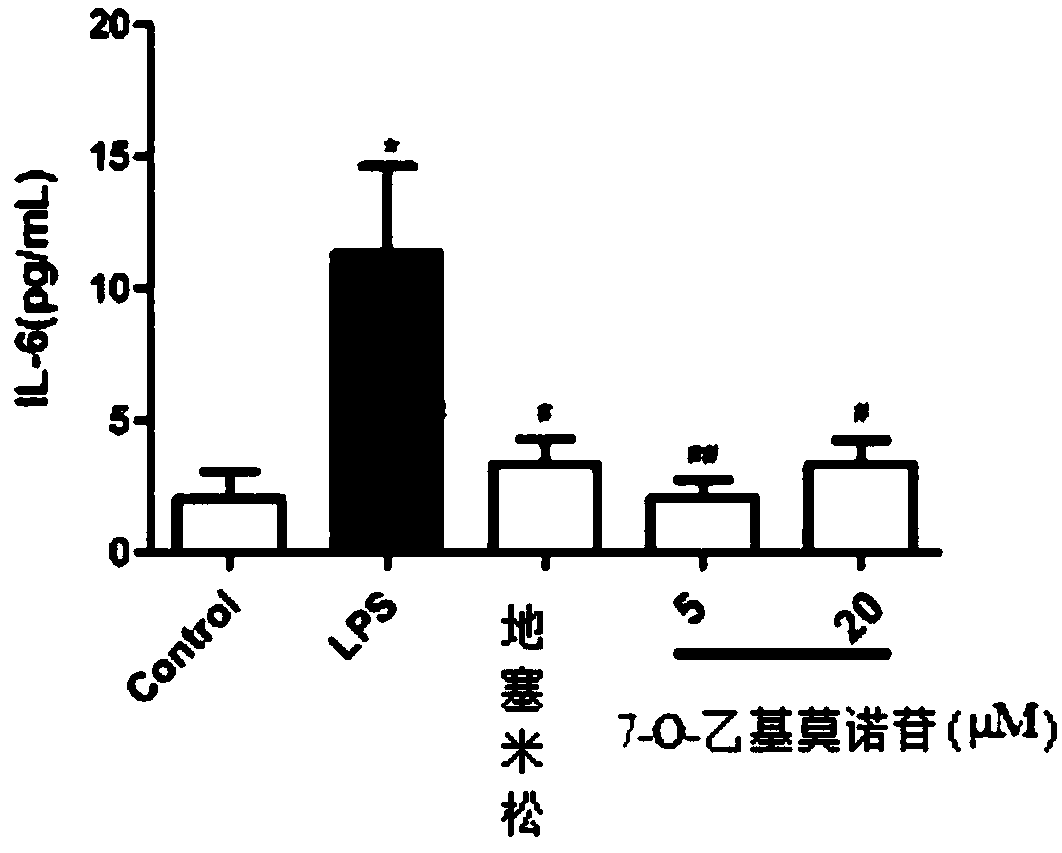
The preparation method of 7-o-ethyl morroniside
A technology of ethyl morronoside and ethanol, applied in the field of medicine, can solve the problems of low content, separation and purification can not well satisfy pharmacological activity research, no systematic research on pharmacological activity, etc., and achieves less impurities and reaction technology. Reasonable design and low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The preparation method of 7-O-ethyl morroniside, comprises the following steps:
[0028] (1) Dissolving: Weigh 1 kg of the total iridoid glycosides of Corni officinalis (containing 40% morroniside and 20% loganin), add 10L of absolute ethanol, and stir at room temperature to disperse it evenly;
[0029] (2) adjust the pH value: add an appropriate amount of dilute phosphoric acid to adjust the pH=3;
[0030] (3) Heating reaction: reflux at 80°C for 16 hours; filter to obtain extract, concentrate to obtain extract;
[0031] (4) Purification and crystallization: add ethyl acetate to the extract to dissolve, filter out the insoluble matter, concentrate the filtrate to a small volume, add dichloromethane to the concentrated solution and stir until there is precipitation, leave to crystallize, and then use ethyl acetate to dissolve the crystallization The ester was dissolved, recrystallized by adding dichloromethane, filtered and dried under reduced pressure to obtain 365 g ...
Embodiment 2
[0033] The preparation method of 7-O-ethyl morroniside, comprises the following steps:
[0034] (1) Dissolving: Weigh 1 kg of total iridoid glycosides of Cornus officinalis (containing 40% morroniside and 20% loganin), put it into an extraction tank, add 15L of absolute ethanol, and stir under normal temperature to make it evenly dispersed;
[0035] (2) adjust the pH value: add an appropriate amount of dilute sulfuric acid to adjust the pH=2;
[0036] (3) Heating reaction: reflux at 90°C for 16 hours; filter to obtain extract, concentrate to obtain extract;
[0037] (4) Purification and crystallization: add ethyl acetate to the extract to dissolve, filter to remove insoluble matter, concentrate the filtrate to a small volume, add dichloromethane to the concentrated solution and stir until there is precipitation, leave to crystallize, and then use ethyl acetate to dissolve the crystallization The ester was dissolved, recrystallized by adding dichloromethane, filtered and dried...
Embodiment 3
[0039] The preparation method of 7-O-ethyl morroniside, comprises the following steps:
[0040] (1) Dissolving: Weigh 1 kg of the total iridoid glycosides of Corni officinalis (containing 40% morroniside and 20% loganin), add 20L of absolute ethanol, and stir at room temperature to disperse it evenly;
[0041] (2) adjust the pH value: add an appropriate amount of dilute hydrochloric acid to adjust the pH=1;
[0042] (3) Heating reaction: reflux extraction at 100°C for 16 hours; filter to obtain extract, concentrate to obtain extract;
[0043] (4) Purification and crystallization: add ethyl acetate to the extract to dissolve, filter out the insoluble matter, concentrate the filtrate to a small volume, add dichloromethane to the concentrated solution and stir until there is precipitation, leave to crystallize, and then use ethyl acetate to dissolve the crystallization Dissolve the ester, add dichloromethane for recrystallization, filter and dry under reduced pressure to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com