Preparation method for glycerophosphatidylcholine
A technology of glycerol phosphatidyl choline and ion exchange resin is applied in the field of preparation of glycerol phosphatidyl choline, and can solve the problems of complex process, high cost, unqualified product optical rotation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
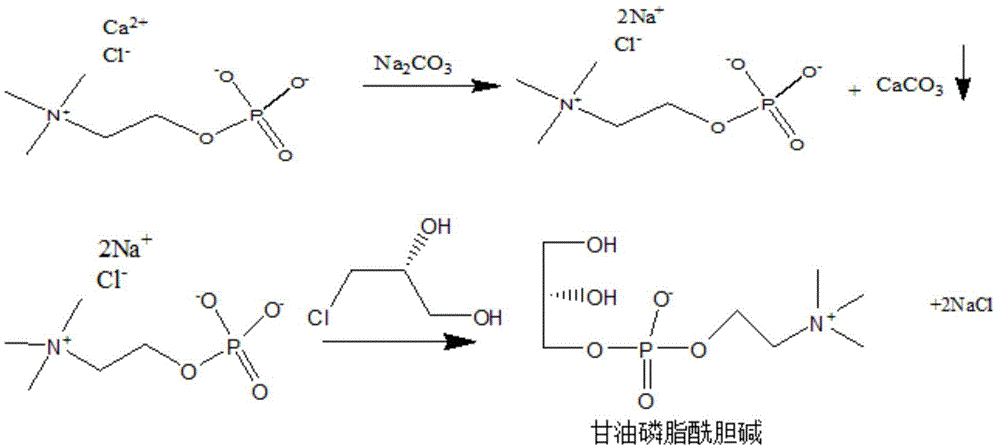
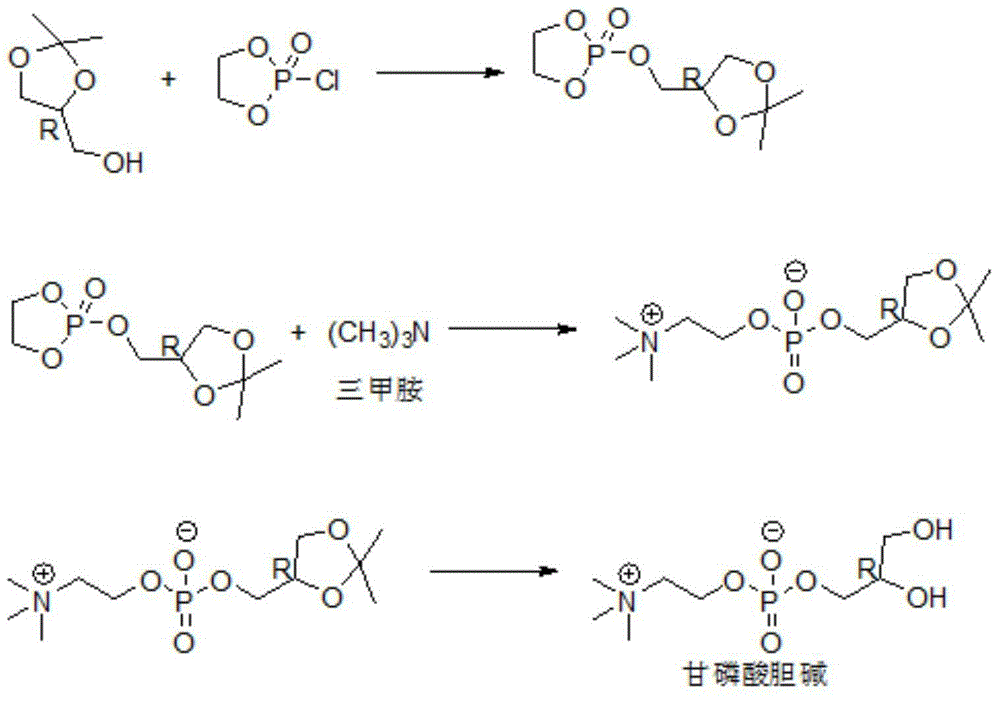
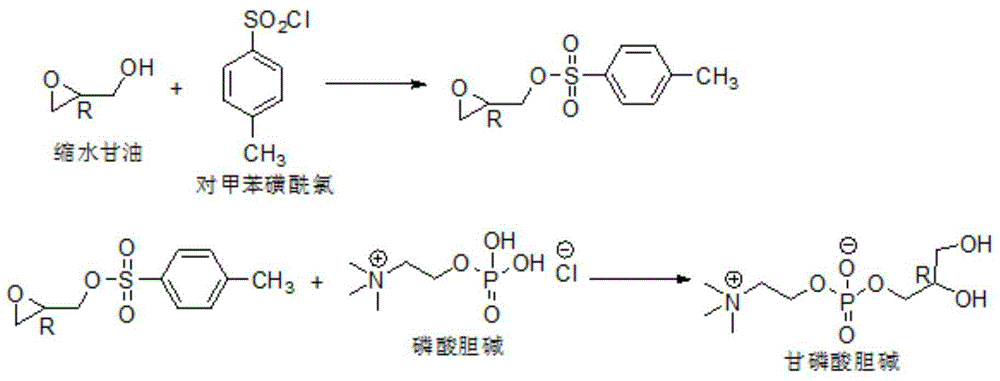
[0034] The present invention provides a kind of preparation method of glycerol phosphatidylcholine, wherein, said preparation method comprises:
[0035]1) mixing the compound shown in formula (I) with anhydrous sodium carbonate to prepare the mixture M1;
[0036] 2) Reflux the mixture M1 and (R)-(-)-3-chloro-1,2-propanediol in absolute ethanol to obtain the mixture M2;
[0037] 3) Dilute the mixture M2 by 5-10 times and flow through the first ion exchange resin to obtain the mixture M3;
[0038] 4) Dilute the mixture M3 by 2-4 times and flow through the second ion exchange resin to obtain the mixture M4;
[0039] 5) Washing the mixture M4 with ethanol with a concentration of 70-90% by weight to obtain the mixture M5;
[0040] 6) Washing the mixture M5 with distilled water to obtain the mixture M6;
[0041] 7) Pass the mixture M6 through the third ion exchange resin and then filter the filtrate to obtain glycerol phosphatidylcholine;
[0042]
[0043] Among them, R is Ca...
preparation example 1
[0057] 1) At 25°C and -0.09MPa, slowly add 140g of phosphoric acid dropwise (the drop rate is 10 drops / s) to 100g of choline chloride crude product (containing 70% choline chloride), after the dropwise addition is completed The temperature was raised to 70°C for vacuum distillation until there was no distilled water, then the temperature was raised to 120°C and the pressure was controlled at -0.09MPa to react for 6h, and then 50g of water was added to terminate the reaction to obtain a phosphorylcholine chloride mixture;
[0058] 2) At 25° C., mix 100 g of the above-mentioned phosphorylcholine chloride mixture with 3000 g of calcium hydroxide aqueous solution (the mass fraction of calcium hydroxide is 15%) for 10 min until the pH of the system reaches 8 to obtain a metathesis mixture;
[0059] 3) At 25°C and 35Pa, the above-mentioned metathesis mixture was subjected to vacuum distillation until the solid was precipitated to obtain a solid-liquid mixture;
[0060] 4) Cool the a...
preparation example 2
[0063] 1) At 108°C, 100 g of (S)-3-chloro-1,2-propylene oxide, 60 g of water and 1.5 g of sulfuric acid were subjected to a ring-opening reaction for 6 hours to obtain a ring-opening mixture;
[0064] 2) At 25° C., 15 g of aqueous sodium hydroxide solution (the mass fraction of sodium hydroxide is 5%) and 100 g of the ring-opened mixture were neutralized for 15 minutes to obtain a neutralized mixture (pH 7.0);
[0065] 3) remove the water in the neutralized mixture by distillation under reduced pressure (the water content of the mixture after water removal is 0.3%), and then filter to obtain the filtrate;
[0066] 4) The filtrate was subjected to fractional distillation at 33pa and 126°C to obtain (R)-3-chloro-1,2-propanediol.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com