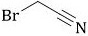
Preparation method of bromoacetonitrile
A technology of bromoacetonitrile and bromide salt, which is applied in the field of preparation of bromoacetonitrile, can solve the problems of poor working environment, low product content, large phosphorus pentoxide and three wastes, etc., to avoid the production of hydroxyacetonitrile, product purity and yield High, short response time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] A kind of preparation method of bromoacetonitrile, it is carried out according to the following steps:
[0049] Put the bromide salt 3 and the solvent into the reaction flask, heat up to 50-90 ° C, remove part of the solvent under reduced pressure to remove the water in the system; remove the vacuum with nitrogen, cool down to 30 ± 2 ° C, add cyanomethylsulfonic acid Acetate 2; the addition is completed, and the temperature is slowly raised to 100-120 ° C, and the crude product of bromoacetonitrile is separated by vacuum distillation through the method of reactive distillation, while carrying out chemical reaction; the crude product is rectified under vacuum to obtain high purity The compound bromoacetonitrile 1;
[0050] The reaction formula is as follows:
[0051] ;
[0052] In compound 2, R represents a straight or branched chain alkyl group having up to 4 carbon atoms, a straight chain or branched chain haloalkyl group having up to 10 carbon atoms and up to 21 h...
Embodiment 1
[0057] A kind of preparation method of bromoacetonitrile
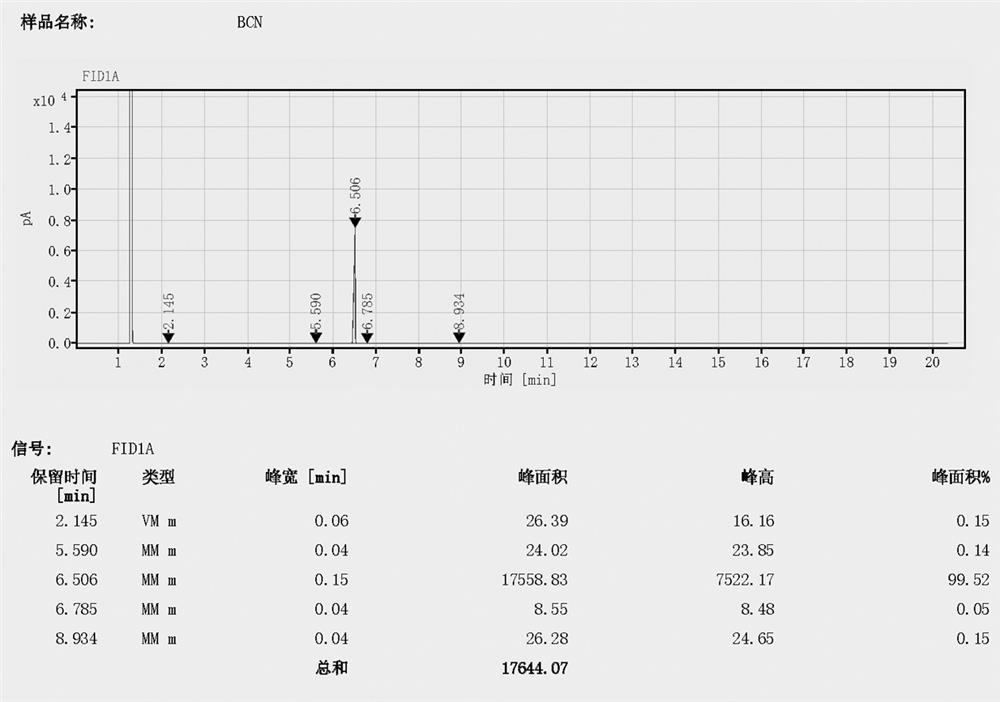
[0058]In a 1000mL reaction flask, put 120g of anhydrous sodium bromide and 300g of sulfolane, heat up to 90°C, remove about 10g of solvent under reduced pressure; remove the vacuum with nitrogen, cool down to 30±2°C, add 135g of cyanomethylmethanesulfonic acid Acetate; add end, and slowly be warming up to 100-120 ℃, by the method of reactive distillation, while carrying out chemical reaction, carry out vacuum distillation to separate the crude product of bromoacetonitrile; the crude product is rectified under vacuum by water pump, collect 80 -85°C / 40-50mmHg fraction, 102.0 g of product 1 was obtained with 99.52% GC purity and 85.15% yield.
Embodiment 2
[0060] Another preparation method of bromoacetonitrile
[0061] In a 1000mL reaction flask, put 135g of anhydrous sodium bromide and 300g of N-methylpyrrolidone, heat up to 90°C, and remove the water from the system under reduced pressure; remove the vacuum with nitrogen, cool down to 30±2°C, add 210g of cyanide Methyl p-toluenesulfonate; the addition is completed, and the temperature is slowly raised to 100-120 ° C, and the crude product of bromoacetonitrile is separated by vacuum distillation through the method of reactive distillation, while carrying out chemical reaction; After rectification, the fractions at 80-85°C / 40-50mmHg were collected to obtain 97.9g of product 1, the GC purity was 99.58%, and the yield was 81.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com