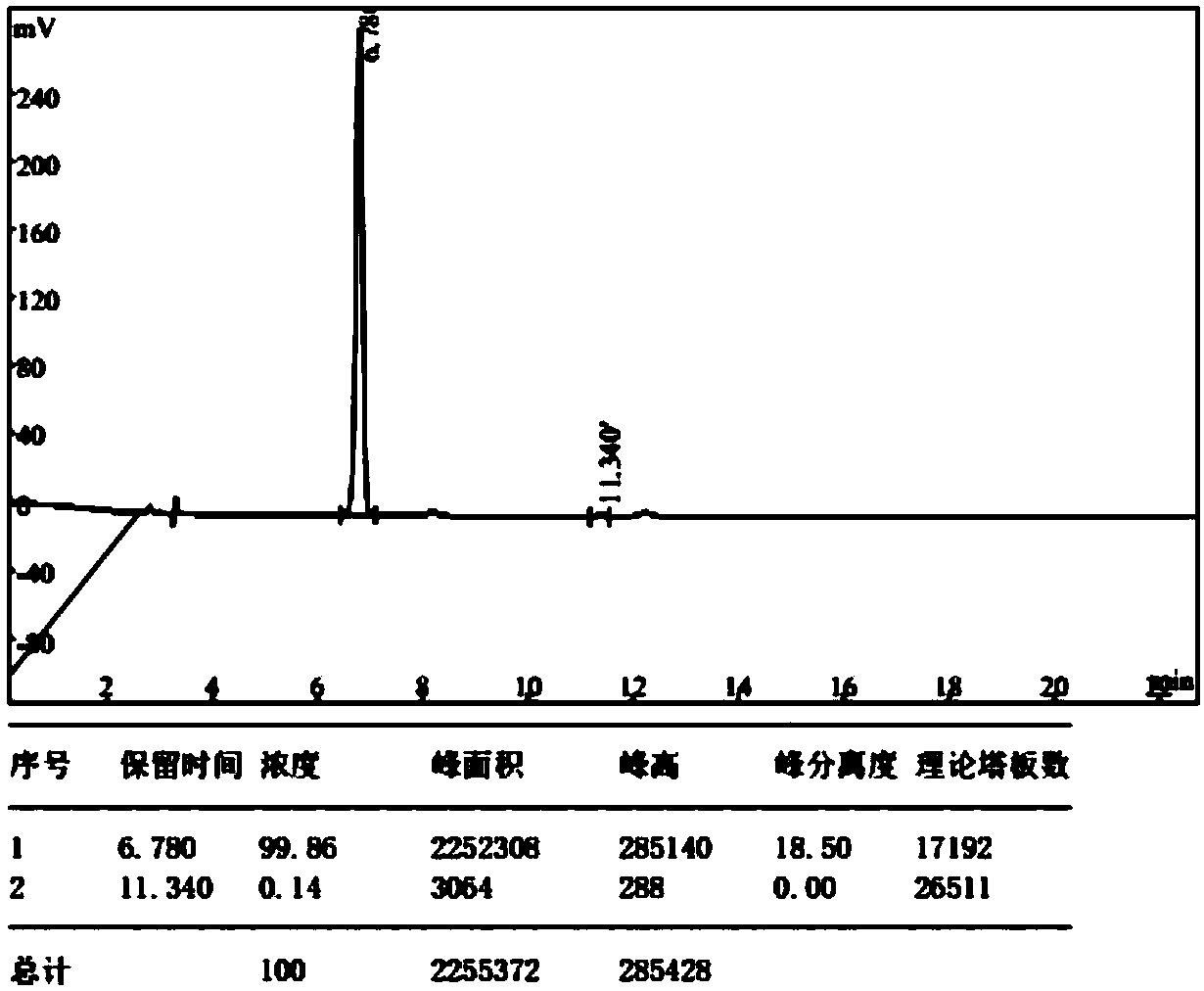
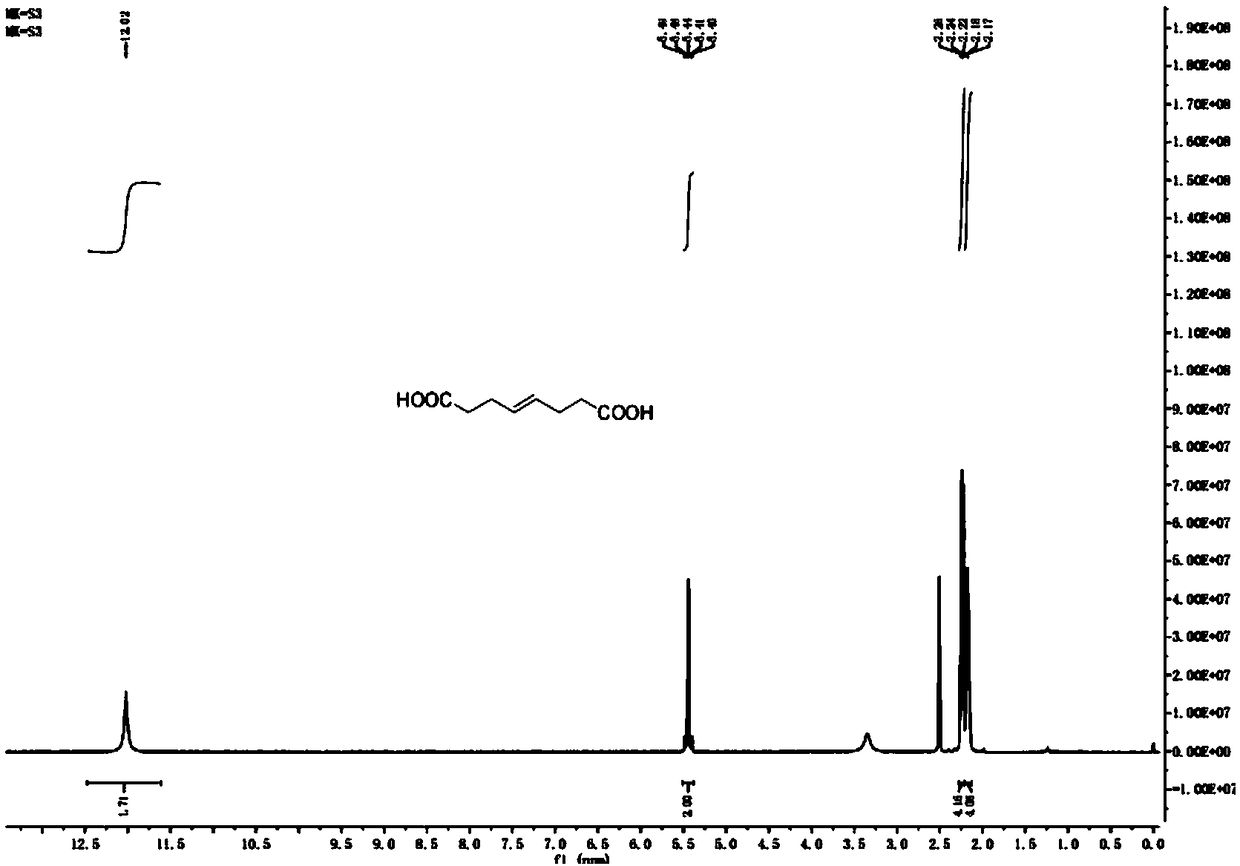
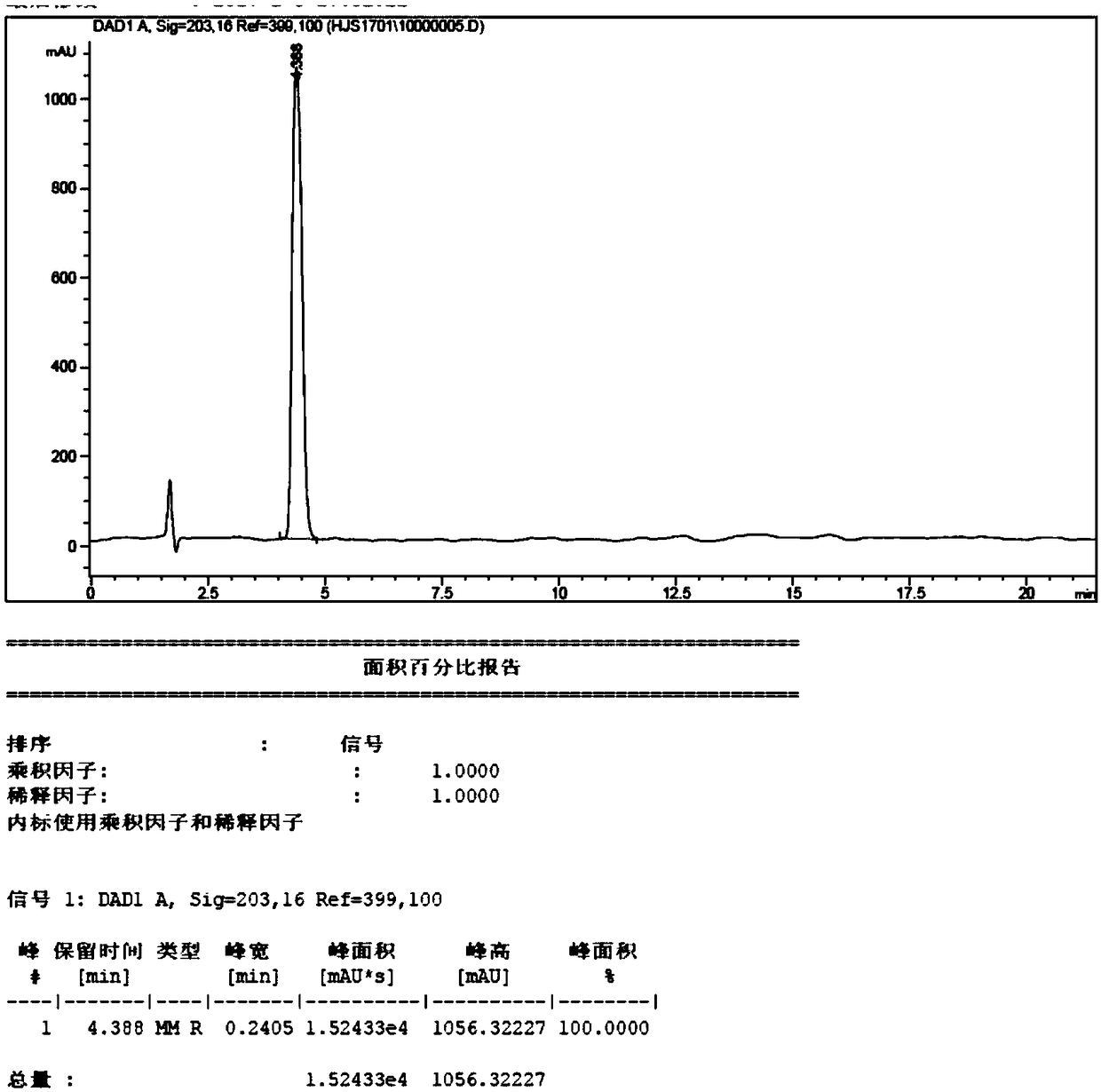
Preparation method of (E)-octyl-4-alkene-1,8-diacid
A technology of diacid and butene is applied in the preparation of carboxylic acid nitrile, the preparation of organic compounds, the preparation of nitrile, etc., to achieve the effects of green process, simple process operation and mild process conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: The preparation method of (E)-oct-4-ene-1,8-dioic acid comprises the following steps:
[0047] step one:
[0048]In a 500ml three-neck reaction flask equipped with a thermometer and a constant pressure dropping funnel, add tetrahydrofuran (THF) (150mL) and Mg powder 11.5g, then raise the temperature of the reaction solution to 40°C, and slowly add 5mL, 2M bromoacetonitrile dropwise therein The reaction was initiated by the THF solution, and then the temperature of the reaction solution was lowered to 10°C, and 70 mL of 2M bromoacetonitrile solution in THF was slowly added dropwise, maintaining the temperature at 10°C, and stirring was continued at 10°C for 1 hour after the addition was completed.
[0049] Then 1,4-dibromo-2-butene (0.047mol) was dissolved in THF (100ml) to prepare a solution of 1,4-dibromo-2-butene, and the prepared A good solution of 1,4-dibromo-2-butene was added dropwise to the above reaction system, and the temperature was kept at 10-...
Embodiment 2
[0052] Embodiment 2: the preparation method of (E)-oct-4-ene-1,8-dioic acid comprises the following steps:
[0053] step one:
[0054] In a 500ml three-necked reaction flask equipped with a thermometer and a constant pressure dropping funnel, add tetrahydrofuran (150ml) and 15g of zinc powder, then raise the temperature of the reaction solution to 40°C, slowly add 5mL, 2M tetrahydrofuran solution of bromoacetonitrile dropwise to initiate the reaction , and then the temperature of the reaction solution was lowered to -10°C, and 50 mL of 2M bromoacetonitrile tetrahydrofuran solution was continued to be slowly added dropwise, the temperature was maintained at 0°C during the dropwise addition, and stirring was continued at 0°C for 1 hour after the dropwise addition was completed.
[0055] Then 1,4-dibromo-2-butene (0.047mol) was dissolved in THF (100ml) to prepare a 1,4-dibromo-2-butene solution, and the prepared Add the 1,4-dibromo-2-butene solution dropwise to the above reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com