Method for preparing bromoacetonitrile from chloroacetonitrile
A technology of chloroacetonitrile and bromoacetonitrile, which is applied in the field of preparing bromoacetonitrile, can solve the problems of difficult treatment of strong acid waste liquid, low reaction yield, high sales price of bromoacetonitrile, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Add 10ml of acetonitrile, 2.00g of chloroacetonitrile, 3.27g of sodium bromide, and 0.39g (0.1eq) of sodium iodide into a 50ml single-necked bottle. Stir and heat up to reflux, and react for 5 hours. The content of bromoacetonitrile was 89%, the content of chloroacetonitrile was 10% by gas phase detection, and there were no other obvious impurities. After the reaction was completed, it was filtered, and the filtrate was concentrated under reduced pressure to recover the solvent, and then rectified under reduced pressure to separate the product to obtain bromoacetonitrile with a yield of 78%.
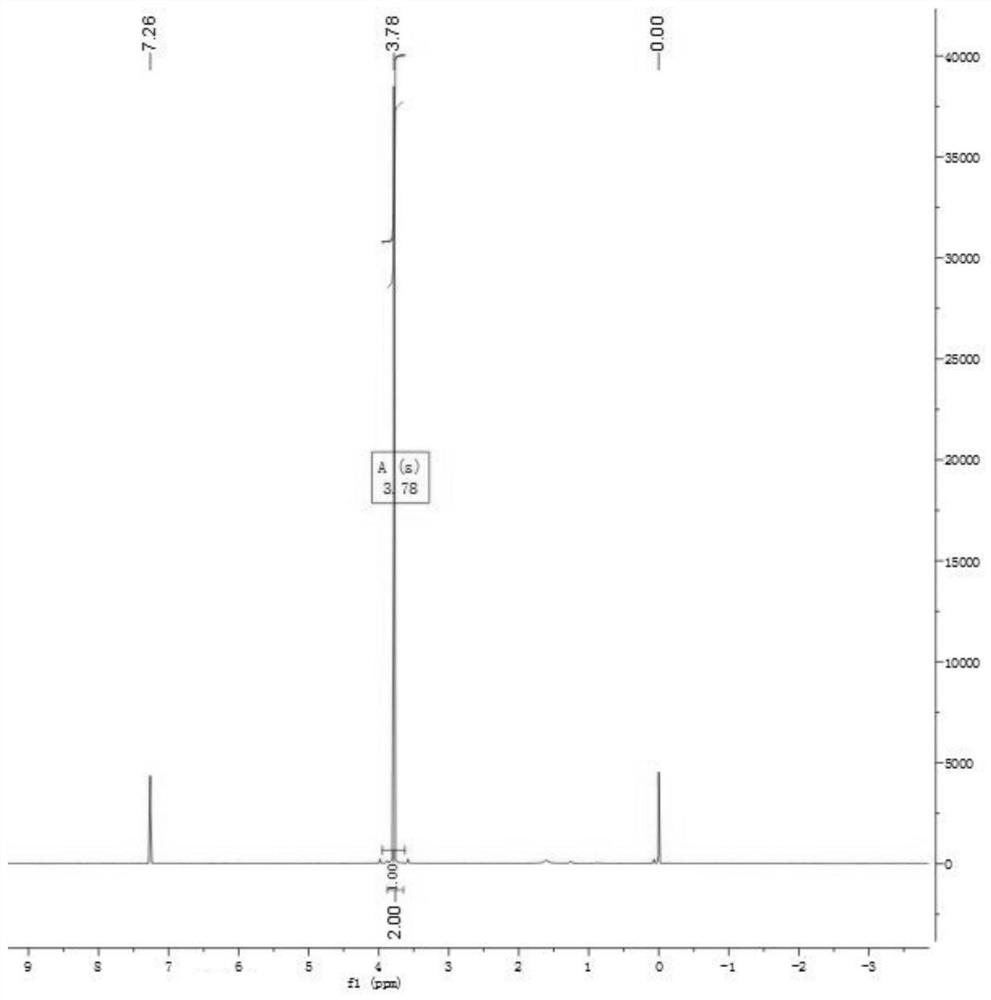
[0023] 1 H NMR(400MHz,Chloroform-d)δ3.78(s,2H).
[0024] Gas phase detection method:
[0025] Chromatographic column: DB-624 (30m×0.32mm, 1.8μm)
[0026] Column temperature: the initial column temperature is 50°C, rise to 200°C at 10°C / min, and keep for 10min
[0027] Injection port: 200°C
[0028] Detector: 220°C
[0029] Injection volume: 0.1μl
[0030] The test solution...
Embodiment 2
[0032] Sodium iodide dosage exploration
[0033] Experimental operation: Add 10mL of acetonitrile, 2.00g of chloroacetonitrile, 3.27g of sodium bromide, and different amounts of sodium iodide into a 50ml single-necked bottle. Stir and warm to reflux. Gas phase detection is carried out in different periods to detect the reaction process and impurity content (mass ratio). The results of the reactions under different conditions are shown in Table 1.
[0034] Table 1 different sodium iodide consumption prepares the reaction result of bromoacetonitrile
[0035]
Embodiment 3
[0037] Reaction solvent exploration: Add 2.00g of chloroacetonitrile, 3.27g of sodium bromide, and 0.08g of sodium iodide into a 50ml single-necked bottle, add 10mL of solvent, stir and raise the temperature to reflux, and react for 5 hours. The solvents investigated include methanol and ethanol, which are regularly sampled and analyzed by gas chromatography. The experimental results are shown in Table 2:
[0038] The reaction result of preparing bromoacetonitrile under the different solvent systems of table 2
[0039]
[0040]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com