A method for preparing 3-nitrile ethyl-2-hydrocarbyl-4h-benzoxazine by bromoacetonitrile under blue light irradiation conditions
A technology of benzoxazine and irradiation conditions, which is applied in the field of functionalization/cyclization reaction of alkenyl-containing compounds, can solve problems such as post-processing troubles, achieve mild reaction conditions, wide applicability, and simple and easy-to-obtain reaction raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
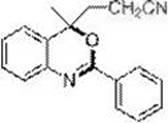
[0023] Embodiment 1: prepare 4-methyl-2-phenyl-4-nitrile ethyl-(4 H )-1,3-Benzoxazine (3a)
[0024] (3-(4-methyl-2-phenyl-4H-benzo[d][1,3]oxazin-4-yl)propanenitrole)
[0025]
[0026] In the dried Schlenk tube, add the amide raw material N-(2-isopropenylphenyl)benzamide (0.1 mmol, 23.7 mg), base: K 3 PO 4 (0.2 mmol, 42.4 mg), catalyst: tris(2-phenylpyridine) iridium Ir(ppy) 3 (0.002 mmol, 1.3 mg), bromoacetonitrile (0.2 mmol, 24 mg), acetonitrile 1 mL, replace the gas in the reaction tube with nitrogen three times, and finally fill the reaction tube with nitrogen, and place the above Schlenk tube in a 16 W blue Stir for 24 h under LED light irradiation. The reaction was terminated, and the reaction solution was quenched with 2 mL of saturated ammonium chloride, and extracted several times with ethyl acetate (4 mL × 5). The organic phases were combined, and the solvent was removed on a rotary evaporator. Finally, it was separated by silica gel column chromatography (eluen...
Embodiment 2
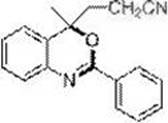
[0028] Embodiment 2: Preparation of 4-methyl-2-phenyl-4-nitrile ethyl-(4 H )-1,3-Benzoxazine (3a)
[0029] (3-(4-methyl-2-phenyl-4H-benzo[d][1,3]oxazin-4-yl)propanenitrole)
[0030]
[0031] In the dried Schlenk tube, add the amide raw material N-(2-isopropenylphenyl)benzamide (0.1 mmol, 23.7 mg), base: K 2 CO 3 (0.2 mmol, 27.6 mg), catalyst: tris(2-phenylpyridine) iridium Ir(ppy) 3 (0.002 mmol, 1.3 mg), bromoacetonitrile (0.2 mmol, 24 mg), acetonitrile 1 mL, replace the gas in the reaction tube with nitrogen three times, and finally fill the reaction tube with nitrogen, and place the above Schlenk tube in a 16 W blue Stir for 24 h under LED light irradiation. The reaction was terminated, and the reaction solution was quenched with 2 mL of saturated ammonium chloride, and extracted several times with ethyl acetate (4 mL × 5). The organic phases were combined, and the solvent was removed on a rotary evaporator. Finally, it was separated by silica gel column chromatograp...
Embodiment 3
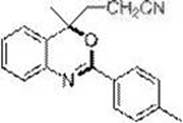
[0032] Embodiment 3: Preparation of 4-methyl-4-nitrile ethyl-2-(p-tolyl)-(4 H )-1,3-Benzoxazine (3b)
[0033] (3-(4-methyl-2-(p-tolyl)-4H-benzo[d][1,3]oxazin-4-yl)propanenitrole)
[0034]
[0035] In the dried Schlenk tube, add the amide raw material N-(2-isopropenylphenyl)-p-benzamide (0.1 mmol, 25.1 mg), base: K 3 PO 4 (0.2 mmol, 42.4 mg), catalyst: tris(2-phenylpyridine) iridium Ir(ppy) 3 (0.002 mmol, 1.3 mg), bromoacetonitrile (0.2 mmol, 24 mg), acetonitrile 1 mL, replace the gas in the reaction tube with nitrogen three times, and finally fill the reaction tube with nitrogen, and place the above Schlenk tube in a 16 W blue Stir for 24 h under LED light irradiation. The reaction was terminated, and the reaction solution was quenched with 2 mL of saturated ammonium chloride, and extracted several times with ethyl acetate (4 mL × 5). The organic phases were combined, and the solvent was removed on a rotary evaporator. Finally, after separation by silica gel column chr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com