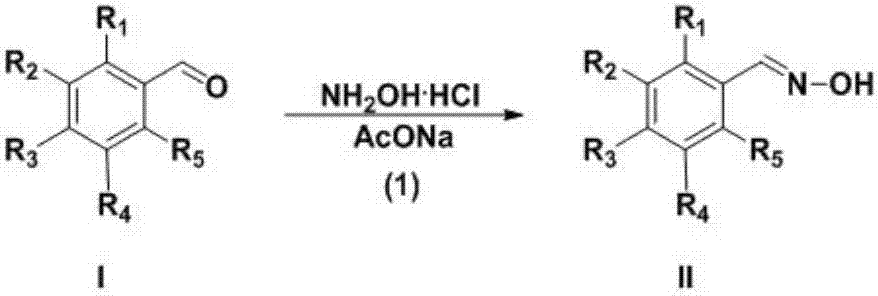
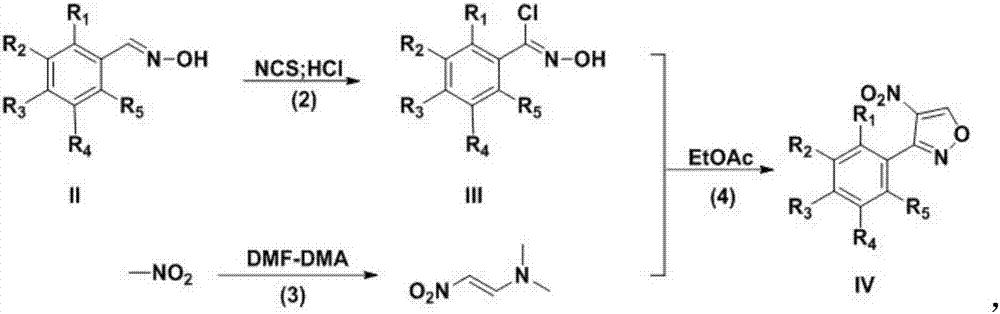
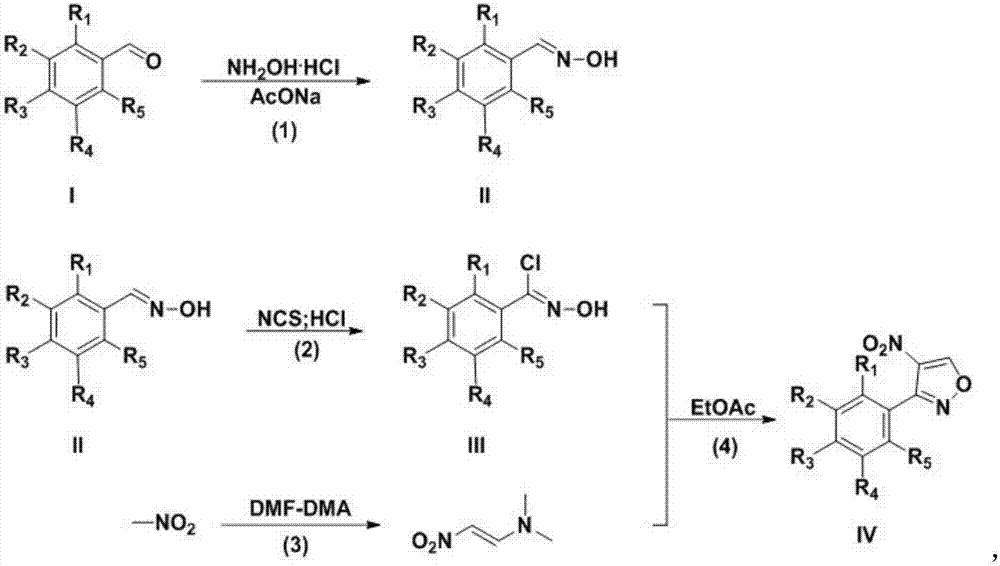
Preparation technology for 3-aryl-4-nitro isoxazole compound
A technology of nitroisoxazole and preparation process, which is applied in directions such as organic chemistry, can solve the problems of high production cost, difficult separation of reaction by-products, and difficulty in obtaining raw materials, and achieves easy product, mild and easy-to-control operating conditions, and low cost of raw materials. Inexpensive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1 Preparation of 3-(4-bromophenyl)-4-nitroisoxazole
[0017] A. Preparation of p-bromobenzaldehyde oxime
[0018] In a single-necked flask, dissolve p-bromobenzaldehyde (10.0g, 54.0mmol), hydroxylamine hydrochloride (4.9g, 70.5mmol) and anhydrous sodium acetate (8.9g, 108.5mmol) in ethanol (100mL), and react at 45°C for 1h . After the reaction was completed, cool to room temperature, filter with suction, wash the filter cake with ethanol (50mL×3), combine the filtrates, evaporate the solvent to dryness under reduced pressure, dissolve the residue with ethyl acetate (100mL), wash with pure water (100mL×2), and remove Dry over sodium sulfate, evaporate the solvent under reduced pressure to obtain 10.0 g of p-bromobenzaldehyde oxime as a white solid, with a yield of 92.8%.
[0019] B. Preparation of Chloroxime
[0020] In a three-neck flask, p-bromobenzaldehyde oxime (5 g, 25.0 mmol) was dissolved in N,N-dimethylformamide (100 mL), NCS (3.3 g, 25.0 mmol) was add...
Embodiment 2
[0026] Example 2 Preparation of 3-(3-bromophenyl)-4-nitroisoxazole
[0027] A. Preparation of m-bromobenzaldehyde oxime
[0028] In a single-necked flask, dissolve m-bromobenzaldehyde (10.0g, 54.0mmol), hydroxylamine hydrochloride (4.9g, 70.5mmol) and anhydrous sodium acetate (8.9g, 108.5mmol) in ethanol (100mL), and react at 45°C for 1h . After the reaction was completed, cool to room temperature, filter with suction, wash the filter cake with ethanol (50mL×3), combine the filtrates, evaporate the solvent to dryness under reduced pressure, dissolve the residue with ethyl acetate (100mL), wash with pure water (100mL×2), and remove Dry over sodium sulfate, and distill off the solvent under reduced pressure to obtain 9.8 g of m-bromobenzaldehyde oxime as a white solid, with a yield of 90.8%.
[0029] B. Preparation of Chloroxime
[0030] In a three-necked flask, m-bromobenzaldoxime (5 g, 25.0 mmol) was dissolved in N,N-dimethylformamide (100 mL), NCS (3.3 g, 25.0 mmol) was ad...
Embodiment 3
[0036] Example 3 Preparation of 3-(2-bromophenyl)-4-nitroisoxazole
[0037] A. Preparation of o-bromobenzaldehyde oxime
[0038] In a single-necked flask, dissolve o-bromobenzaldehyde (10.0g, 54.0mmol), hydroxylamine hydrochloride (4.9g, 70.5mmol) and anhydrous sodium acetate (8.9g, 108.5mmol) in ethanol (100mL), and react at 45°C for 1h . After the reaction was completed, cool to room temperature, filter with suction, wash the filter cake with ethanol (50mL×3), combine the filtrates, evaporate the solvent to dryness under reduced pressure, dissolve the residue with ethyl acetate (100mL), wash with pure water (100mL×2), and remove Dry over sodium sulfate, evaporate the solvent under reduced pressure to obtain 10.1 g of o-bromobenzaldoxime as a white solid, with a yield of 93.4%.
[0039] B. Preparation of Chloroxime
[0040] In a three-neck flask, o-bromobenzaldoxime (5 g, 25.0 mmol) was dissolved in N,N-dimethylformamide (100 mL), NCS (3.3 g, 25.0 mmol) was added in batche...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com