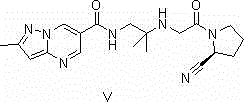
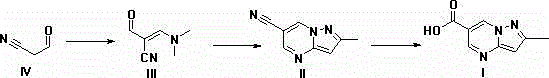
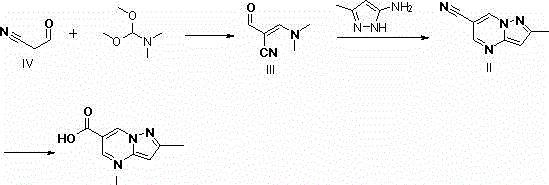
Synthesis method of Anagliptin key intermediate
A synthetic method and intermediate technology, applied in the field of drug synthesis, can solve the problems of less reported synthetic routes of pyrimidine-6-carboxylic acid, and achieve the effects of environmental friendliness, high product purity, and less side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 69.06g (1.0mol) of cyanoacetaldehyde, dissolved in 300ml of methanol, added 132g (1.1mol) of N,N-dimethylformamide dimethyl acetal, reacted at 20-25°C for 20-25h, and reduced Concentrate under reduced pressure to obtain an orange-yellow solid, which is recrystallized from ethanol to obtain a light yellow crystal, which is air-dried at 50°C for 10 hours to obtain 87 g of a solid, with a yield of 70%.
Embodiment 2
[0044] 69.06g (1.0mol) of cyanoacetaldehyde, dissolved in 400ml of ethanol, added 132g (1.1mol) of N,N-dimethylformamide dimethyl acetal, reacted at 20-25°C for 20-25h, and reduced Concentrate under reduced pressure to obtain an orange-yellow solid, which is recrystallized from ethanol to obtain light-yellow crystals, and air-dried at 50°C for 10 hours to obtain 75 g of solid, with a yield of 61.3%.
Embodiment 3
[0046] 69.06g (1.0mol) of cyanoacetaldehyde, dissolved in 550ml of isopropanol, add 132g (1.1mol) of N,N-dimethylformamide dimethyl acetal, react at 20-25℃ for 20-25h, end Concentrate under reduced pressure to obtain an orange-yellow solid, which is recrystallized from ethanol to obtain a light yellow crystal, which is air-dried at 50°C for 10 hours to obtain 76 g of a solid, with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com