Patents
Literature
92 results about "CMN pyrazole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
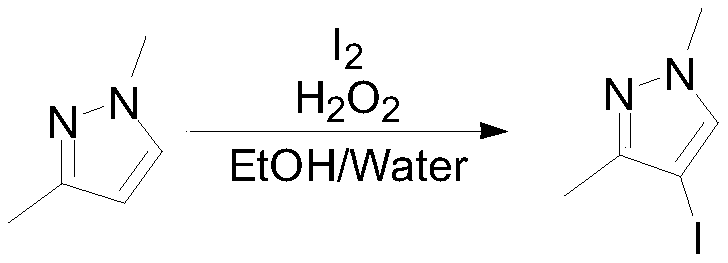
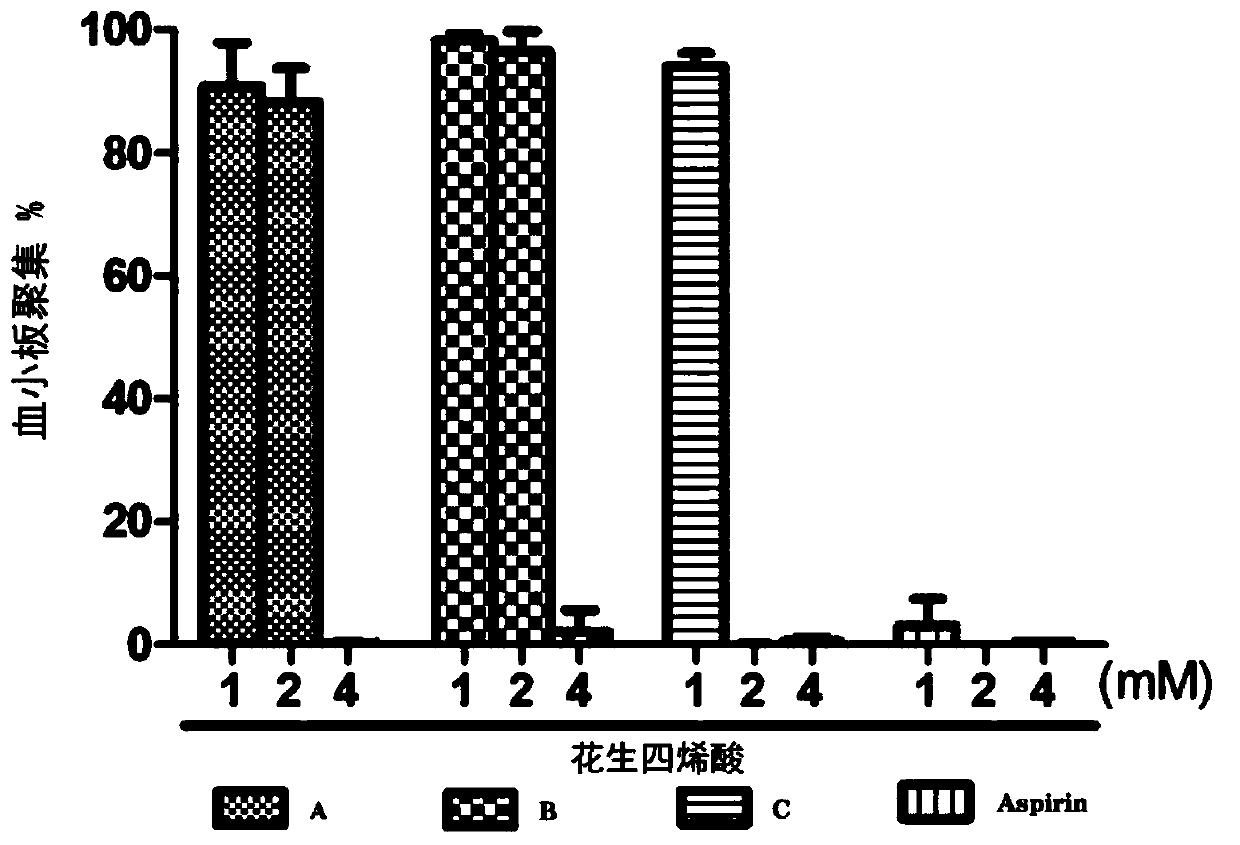
Methyl jasmonate and CMN-pyrazole applied alone and in combination can cause mature orange abscission ☆
Solid forms of an azocyclic amide
Disclosed are solid forms of 1-[4-[4-[5-(2,6-difluorophenyl)-4,5-dihydro-3-isoxazolyl]-2-thiazolyl]-1-piperidinyl]-2-[5-methyl-3-(trifluoromethyl)-1H-pyrazol-1-yl]ethanone (Compound 1). Methods for the preparation of solid forms of Compound 1 and for the conversion of one solid form of Compound 1 into another are disclosed.Disclosed are fungicidal compositions comprising a fungicidally effective amount of a solid form of Compound 1 and at least one additional component selected from the group consisting of surfactants, solid diluents and liquid carriers. Compositions comprising a mixture of a solid form of Compound 1 and at least one other fungicide or insecticide are also disclosed.Also disclosed are methods for controlling plant diseases caused by fungal plant pathogens comprising applying to a plant or portion thereof, or to a plant seed, a fungicidally effective amount of a solid form of Compound 1.
Owner:CORTEVA AGRISCIENCE LLC
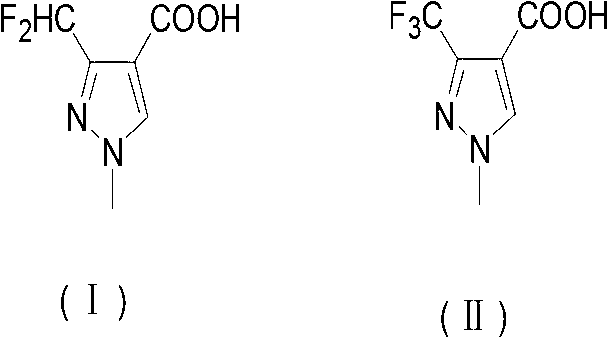
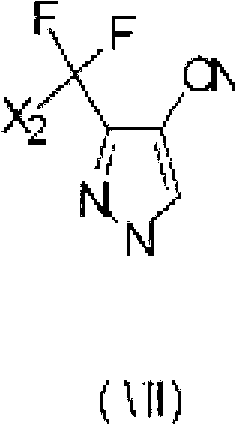
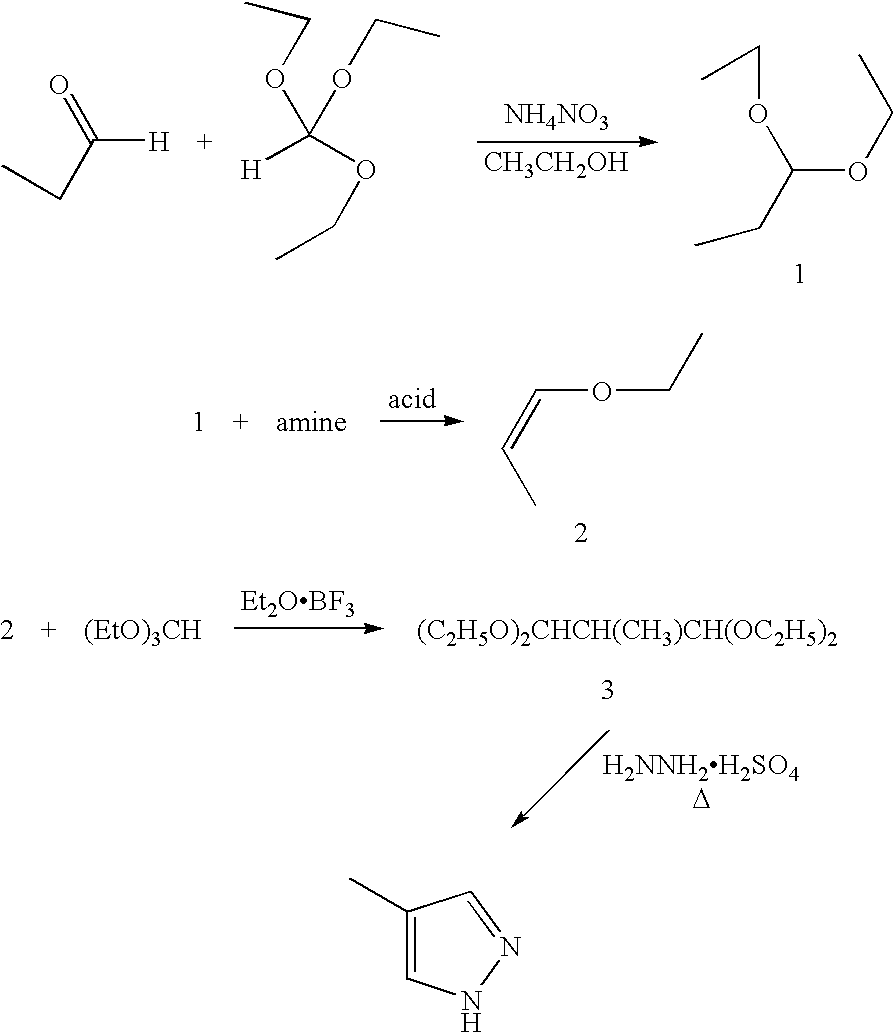
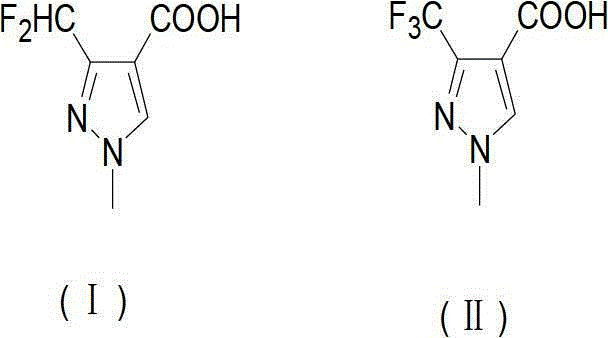
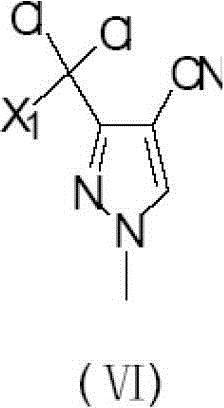
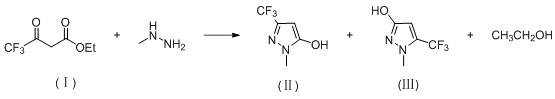
Preparation method of 3-difluoro-methyl pyrazole-4-carboxylic acid and 3-trifluoro-methyl pyrazole-4-carboxylic acid
The invention provides a preparation method of 3-difluoro-methyl-1H-methyl pyrazole-4-carboxylic acid shown in the formula (I) or 3-trifluoro-methyl-1H-methyl pyrazole-4-carboxylic acid shown in the formula (II), and the method comprises the following steps of: (a) providing a 3-polyfluoro methyl-1H-pyrazole-4-nitrile group shown in the formula (VII); and (b) under the condition of participation of acid or alkaline, carrying out hydrolysis reaction on the 3-polyfluoro methyl-1H-pyrazole-4-nitrile group shown in the formula (VII), thus obtaining the 3-difluoro-methyl-1H-methyl pyrazole-4-carboxylic acid shown in the formula (I) or 3-trifluoro-methyl-1H-methyl pyrazole-4-carboxylic acid shown in the formula (II).
Owner:LANZHOU CHEMSPECWEIER CHEM CO LTD
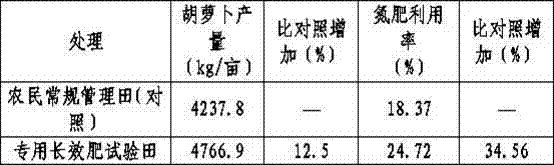
Novel sustained-release long-acting compound fertilizer and preparation method thereof
ActiveCN103936493AGood conversion effectLow costAgriculture gas emission reductionFertilizer mixturesSoil scienceBoronic acid
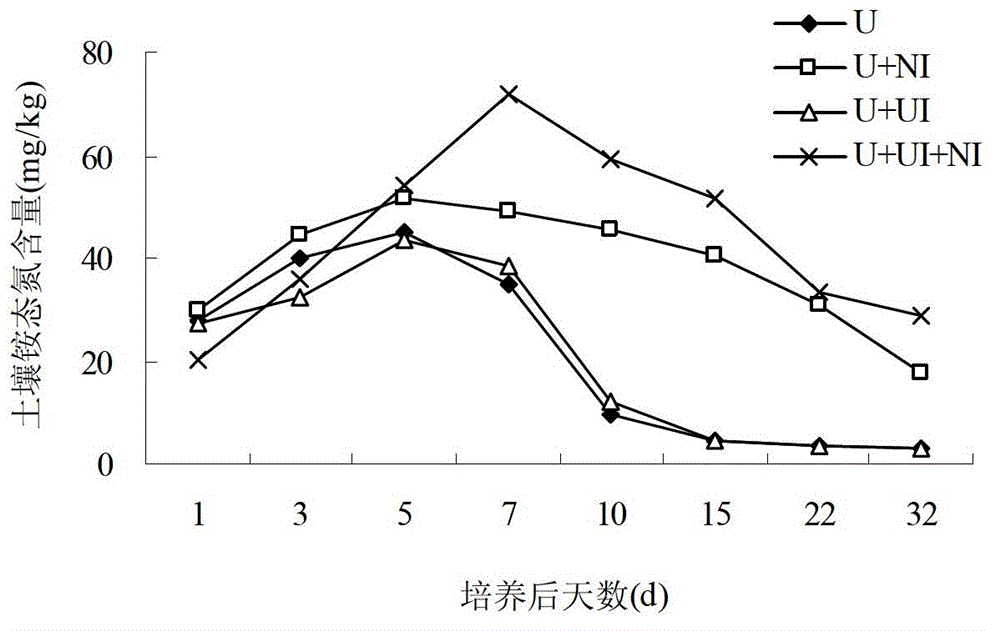
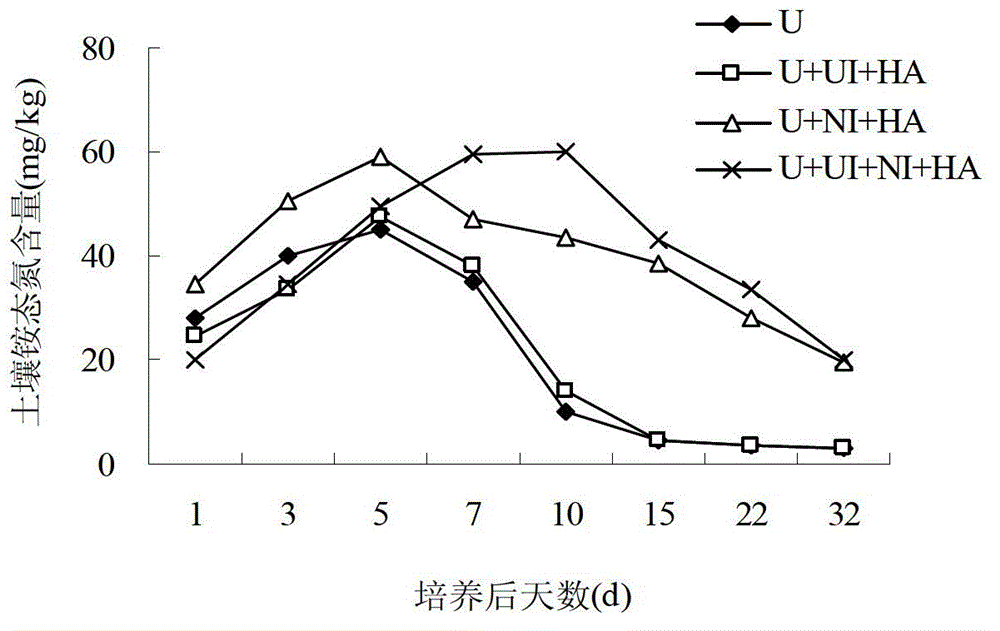
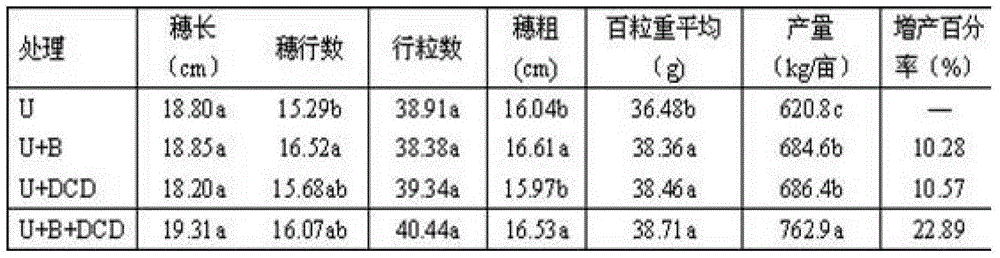
The invention relates to a sustained-release long-acting fertilizer, in particular to a stable fertilizer capable of achieving sustained release and long acting by regulating the conversion process of mineral elements in soil by a novel composite regulating agent. The sustained-release long-acting fertilizer is prepared by mixing a compound fertilizer containing amide nitrogen or ammonium nitrogen with the novel composite regulating agent -in a certain proportion. The novel composite regulating agent is formed by compounding three types of substances in a proper proportion, wherein borax or boric acid simultaneously serves as a synergist for a urease inhibitor and a nitrification inhibitor, and as a plant essential nutrient element; dicyandiamide, 3,5-dimethyl pyrazole, 2-chloro-6-(trichloromethyl) pyridine or the like serve as the nitrification inhibitor; and a humic acid substance serves as the synergist. Compared with a traditional compound (composite) fertilizer, after the fertilizer added with the novel composite regulating agent is applied into soil, the conversion of the nutrient of the fertilizer in soil can be effectively regulated, furthermore the nutrient release period can be effectively prolonged, the nutrient utilization rate can be improved, and environment pollution can be reduced.
Owner:辽宁盛源肥业科技有限公司
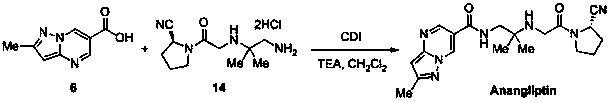
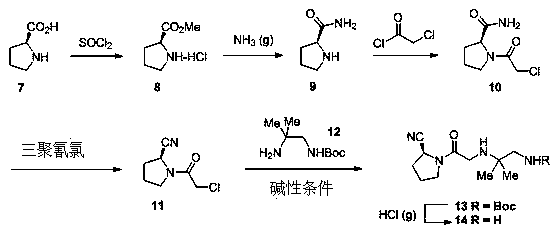
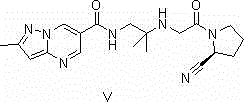
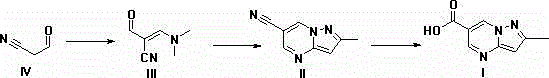
Synthesis method of anagliptin
InactiveCN105503878AHigh reaction yieldEasy post-processingOrganic chemistryBulk chemical productionDiabrezideNucleophilic substitution
The invention relates to a synthesis method of bulk drug of anagliptin for treating II type diabetes, and aims to solve the problem that at present there is no industrial synthesis method of anagliptin. The synthesis method comprises the following steps: (1) taking vinyl ethyl ether and trichloroacetic chloride as the raw materials, carrying out three-step reactions to obtain an intermediate (4) with a protected aldehyde group; carrying out dehydration condensation between the intermediate (4) and 3-amino-5-methylpyrazole to obtain pyrazolopyrimidine parent nucleus; and hydrolyzing carboxyl ethyl ester to obtain 2-methyl-pyrazolo[1,5-a]pyrimidine-6-carboxylic acid6; (2) taking L-proline as the raw material, subjecting L-proline to methyl esterification, ammoniation, acetylation, and cyaniding reactions to obtain a chiral cyanopyrrole intermediate (11); making the chiral cyanopyrrole intermediate (11) and a diamine segment (12) carry out nucleophilic substitution reactions under an alkaline condition to obtain an intermediate (13), and finally removing the Boc protective group from the intermediate (13) in the presence of hydrochloric acid to obtain a cyanopyrrole amine intermediate (14); (3) coupling 2-methyl-pyrazolo[1,5-a]pyrimidine-6-carboxylic acid 6 with the cyanopyrrole amine intermediate (14) under condensation conditions so as to obtain the bulk drug anagliptin.
Owner:南通佰康生物医药有限公司
Special slow-release long-acting compound fertilizer for carrot and application method for same
ActiveCN103360150AMeet nutrient needsThe formula is scientific and reasonableFertilising methodsAgriculture gas emission reductionThio-Phosphate
The invention relates to a special slow-release long-acting compound fertilizer for carrot and an application method for the special slow-release long-acting compound fertilizer. The fertilizer is composed of active ingredients and auxiliary materials, wherein the active ingredients comprise, in parts by weight, 20-25 parts of nitrogen, 8-12 parts of phosphorus (P2O5), 14-18 parts of potassium (K2O), and an inhibitor with an addition amount which is 2-4% of the dosage of nitrogen in the compound fertilizer, wherein the inhibitor is a composite inhibitor composed of a urease inhibitor and a nitrification inhibitor at a weight ratio of 1: (2-5); the urease inhibitor is hydroquinone or N-butyl thiophosphoramide; and the nitrification inhibitor is dicyandiamide, 2-chloro-6-(trichloromethyl) pyridine, 2-amino-4-chloro-6-methyl pyridine, 3,4-dimethylpyrazole phosphate, 3,5-dimethylpyrazole or 3,5-dimethylpyrazole phosphate. The fertilizer is capable of meeting the needs of a whole growth period on nutrients by being applied once before carrot planting, high in yield, and excellent in quality.
Owner:山东三方化工集团有限公司
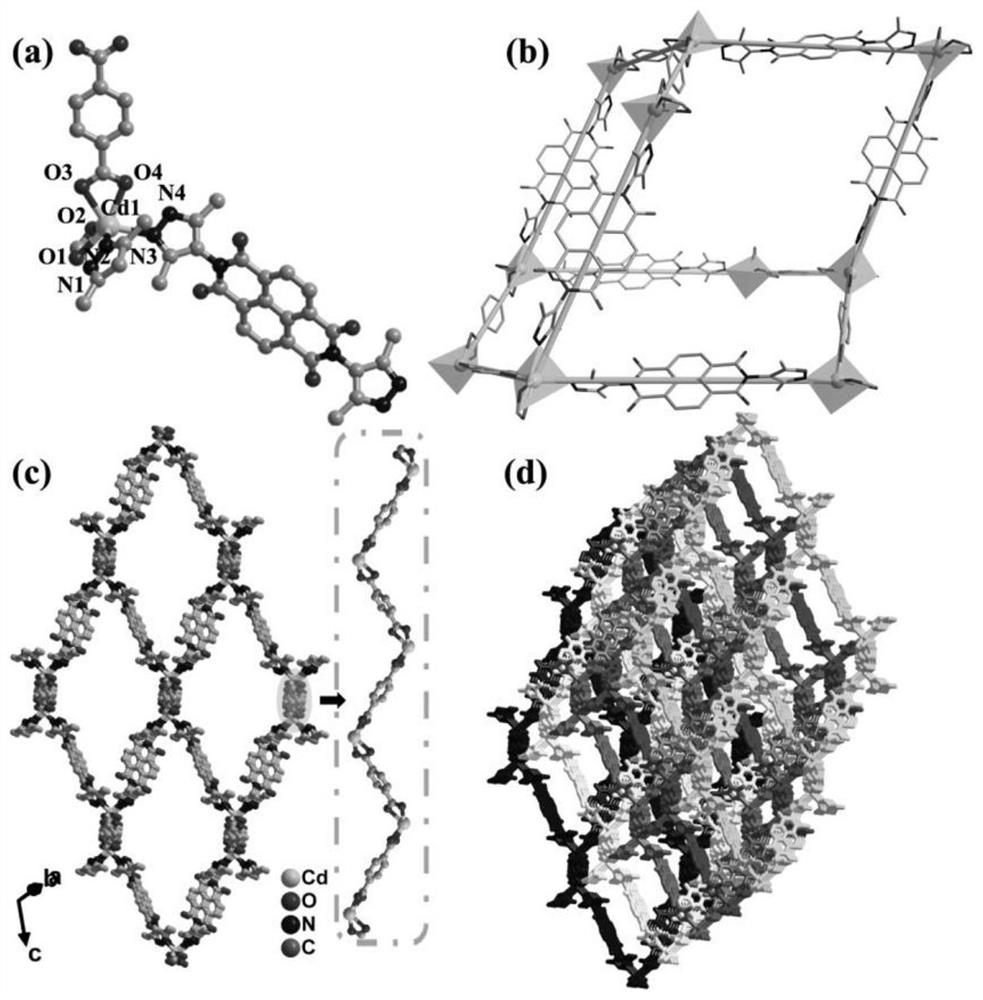
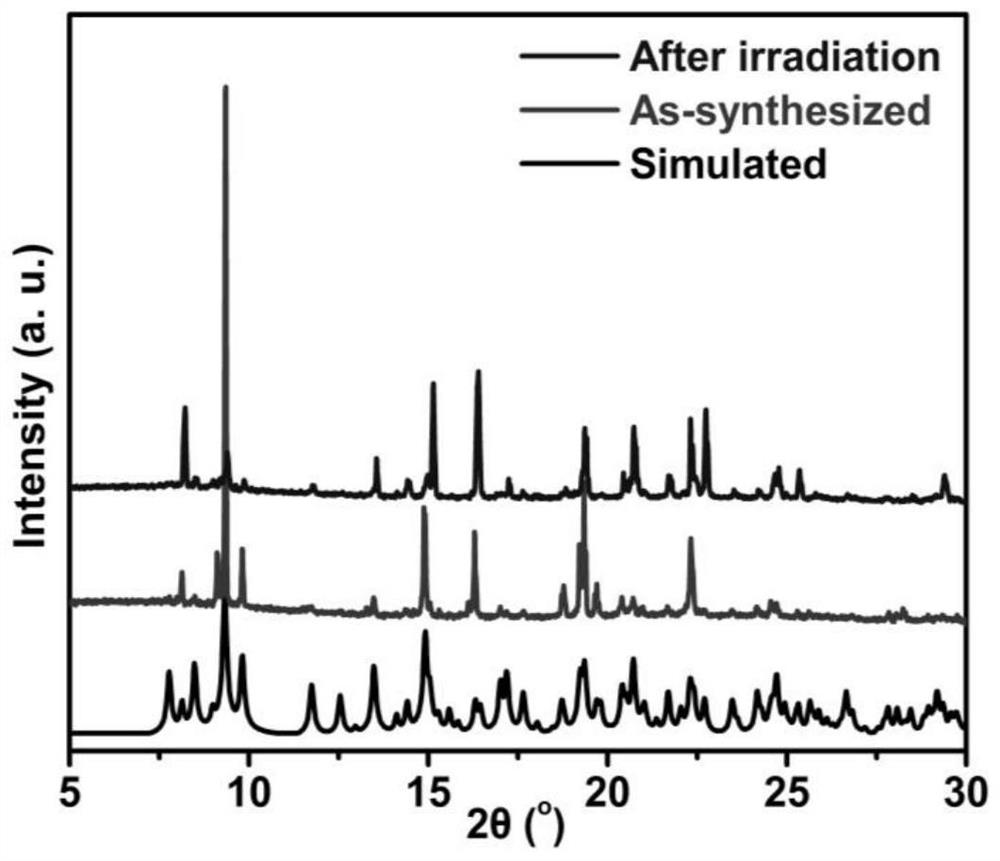
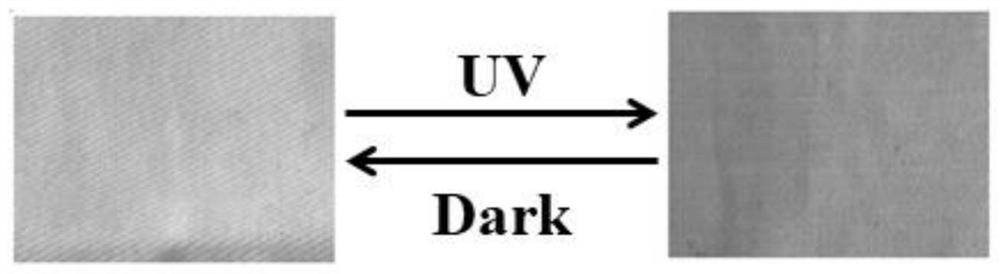
Photochromic naphthalimide Cd-MOF, preparation method thereof, photochromic printing paste and application
ActiveCN112048075ANothing producedMaintain propertiesDyeing processTenebresent compositionsTextile printerToxic gas
The invention discloses a photochromic naphthalimido Cd-MOF, a preparation method thereof, photochromic printing paste and application, and belongs to the field of functional photosensitive fabrics. The molecular formula of the photochromic naphthalimido Cd-MOF is Cd (BDC) (H2NDI). 4H2O, wherein H2NDI is 2, 7-(3, 5-dimethyl- 1H-pyrazole)-1, 4, 5, 8-naphthalene tetracarboxylic diimide, and H2BDC isterephthalic acid. The preparation method disclosed by the invention is simple, feasible and convenient to operate, does not generate toxic gas in the whole process, is green and environment-friendly, conforms to the concept of sustainable development, has no special requirements on equipment, can be used for large-scale production, and has a good and wide development prospect. The photochromic MOFs material is innovatively combined with the traditional textile, so that the characteristics of the textile before and after finishing are kept, the advantages of the MOFs in structure regulation and control can be inherited, and the photochromic behavior and color change of the photosensitive textile are regulated and controlled by utilizing the change of photochromic structure elements.
Owner:YANCHENG INST OF TECH
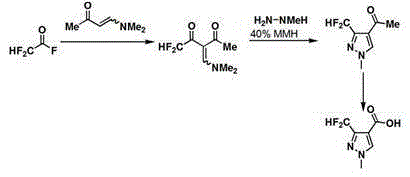
Synthesis method of 3-difluoromethyl-1-methylpyrazole-4-formic acid
InactiveCN106554310ARaw materials are easy to getHigh yieldOrganic chemistrySynthesis methodsHydrazine compound
The invention discloses a synthesis method of 3-difluoromethyl-1-methylpyrazole-4-formic acid, and belongs to the technical field of difluoromethyl pyrazole synthesis. According to the synthesis method, 3-difluoromethyl-1-methylpyrazole-4-formic acid is prepared from 1-dimethylamino-1-butylene-3-one, difluoroacetyl fluoride, and methyl hydrazine through a series of reactions. The provided synthesis method has the advantages of easily available raw materials and high yield, can be applied to industry, and has a comprehensive yield more than 65%.
Owner:陈旭
Synthetic method of 1-methyl 4-pyrazole pinacol ester
InactiveCN102690281AAvoid the disadvantages of being unable to realize industrialized productionSimple processGroup 3/13 element organic compoundsN-ButyllithiumPharmaceutical drug
The invention relates to a synthetic method of 1-methyl 4-pyrazole pinacol ester. The biggest advantage of the invention is that the technology is greatly improved; and the problem that the preparation technology of 1-methyl 4-pyrazole pinacol ester is limited in a laboratory and there is no large-scale production is solved. The technical scheme provided by the invention is as follows: the synthetic method of 1-methyl 4-pyrazole pinacol ester comprises The following steps of: Step 1, performing a reaction between 1-methyl 4-pyrazole and triisopropyl borate under the action of n-butyllithium to obtain an intermediate A; and Step 2, performing a reaction between the intermediate A and pinacol under the action of magnesium sulfate to obtain the target product 1-methyl 4-pyrazole pinacol ester. The obtained 1-methyl 4-pyrazole pinacol ester is an important intermediate commonly-used in pharmaceutical chemistry.
Owner:SHANGHAI SYNTHEALL PHARM CO LTD +2
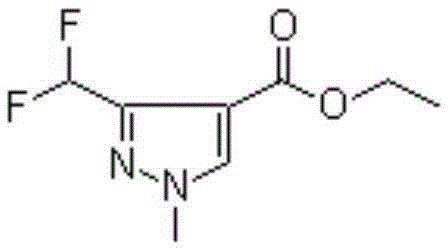
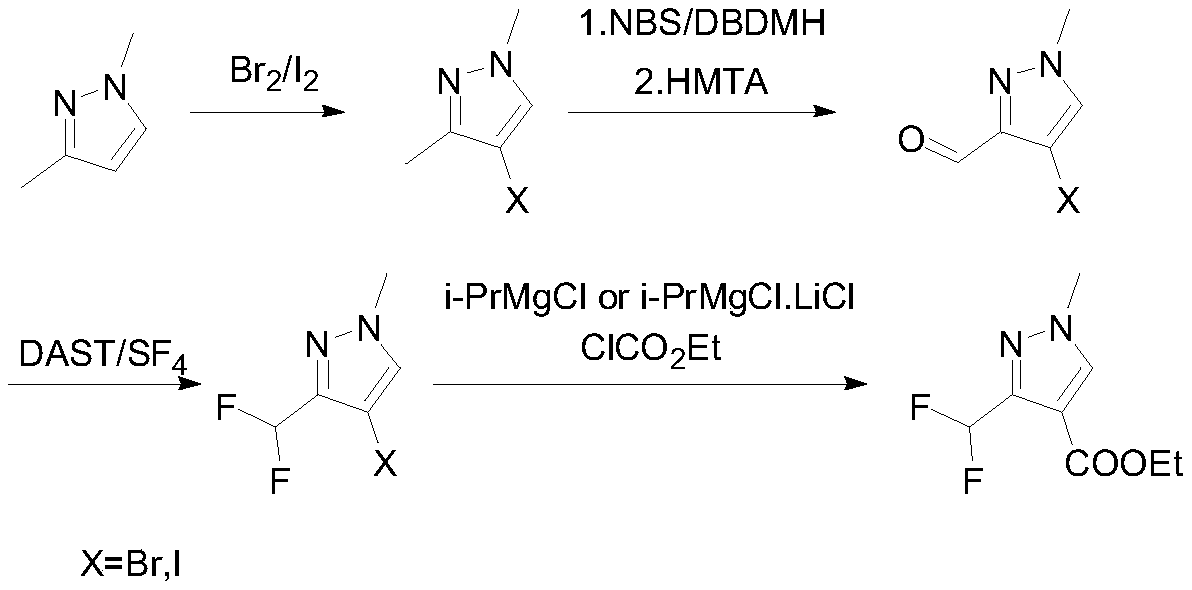
Preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid ethyl ester
ActiveCN111233768AAvoid it happening againSimple methodOrganic chemistryHexamethylenetetramineOrganic synthesis
The invention discloses a preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid ethyl ester, and belongs to the technical field of organic synthesis. The preparation method comprises the following steps: with 1,3-dimethylpyrazole as a raw material, carrying out halogenation to obtain 4-halogenated-1,3,5-tetrahydronaphthalene; carrying out a reaction under the conditions of a bromination reagent and AIBN and hydrolysis with hexamethylenetetramine to obtain 4-halogen-1-methyl-1H-pyrazole-3-formaldehyde, then performing a reaction with a fluorination reagent to obtain 4-halogen-3-difluoromethyl-1-methylpyrazole; finally carrying out a reaction on the 4-halogen-3-difluoromethyl-1-methylpyrazole and a Grignard reagent to obtain 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid ethyl ester. The method is simple and convenient to operate and high in reaction yield, the purity of the obtained product can reach 99.5% or above, and the method has a potential process amplification prospect.
Owner:徐州圣元化工有限公司
Synthesis method of Anagliptin key intermediate
The invention discloses a synthesis method of Anagliptin key intermediate. The synthesis method comprises the following steps: jointing cyanoacetaldehyde with N,N-dimethylformamide dimethyl acetal to obtain (2E)-3-(dimethylamino)-2-formylacrylonitrile, further performing ring closing with 3-amino-5-methylpyrazole to obtain 2-methyl-pyrazolo[1,5-a]pyrimidine-6-carbonitrile, and then hydrolyzing to obtain the Anagliptin key intermediate. The invention relates to a brand-new method for synthesizing 2-methyl-pyrazolo[1,5-a]pyrimidine-6-carboxylic acid, and the method has the advantages of use of raw materials, less side products, high product purity, and low whole cost.
Owner:安徽安腾药业有限责任公司
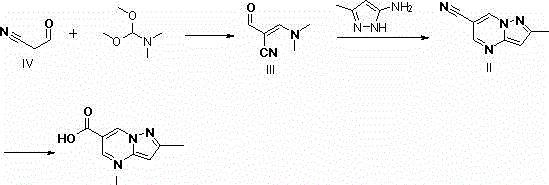
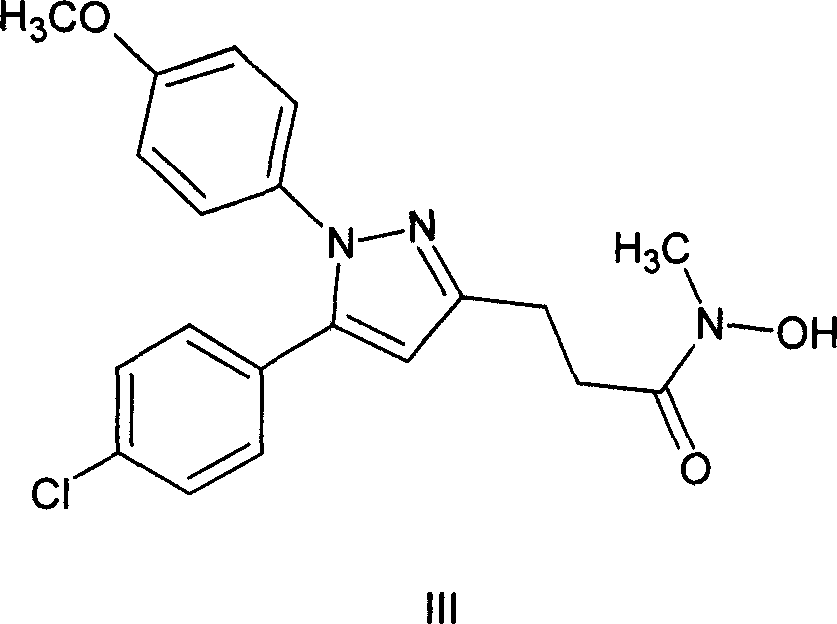
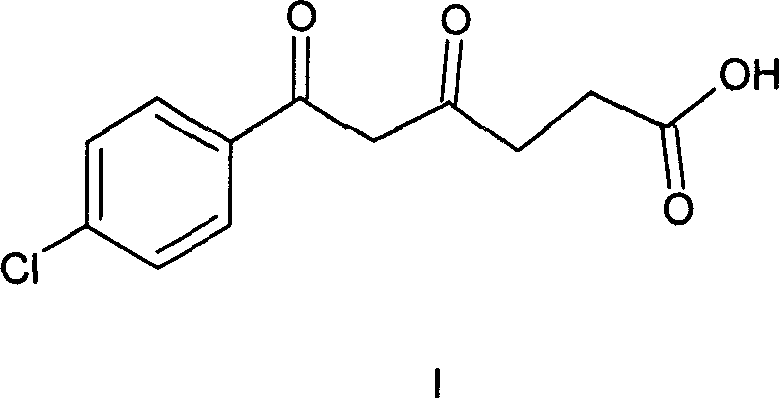
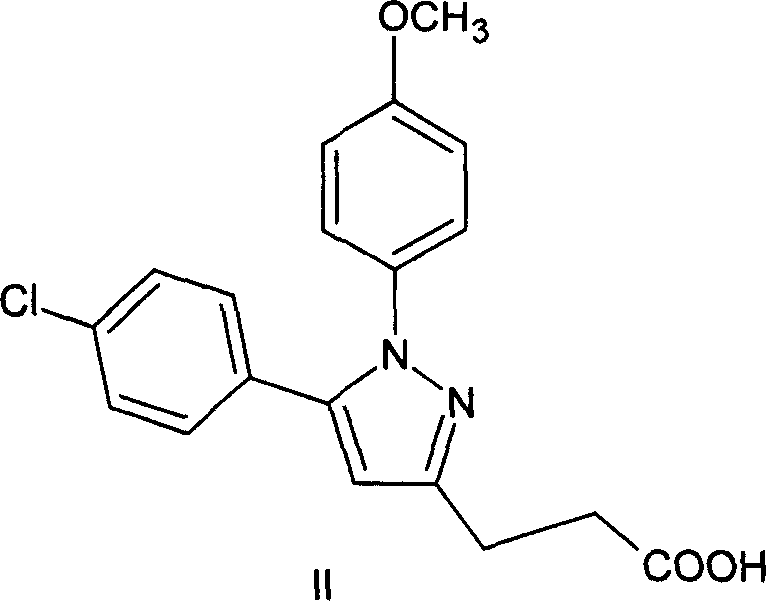
Method of synthesizing 5-(4-chloro-phenyl)-N-hydroxy-1-(4-methoxy-phenyl)-N-methyl-1H-pyrazole-3-propionamide and pharmaceutical use
ActiveCN101012197AEliminate pollutionSimple stepsOrganic active ingredientsOrganic chemistryEpoxyOxygenase
Owner:ZHEJIANG AUSUN PHARMA
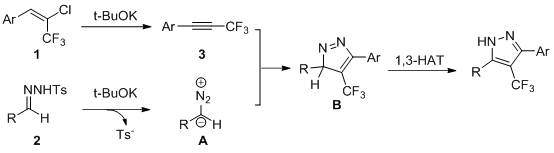
Synthesis method of 3, 5-diaryl-4-trifluoromethyl pyrazole derivative
The invention relates to a synthesis method of a 3, 5-diaryl-4-trifluoromethyl pyrazole derivative, which comprises the following step of: by taking aromatic aldehyde p-toluenesulfonyl hydrazone and 2-chloro-3, 3, 3-trifluoroaryl propylene as reaction substrates, potassium tert-butoxide as alkali and tetrahydrofuran as a solvent, stirring and reacting for 12 hours at 70 DEG C. The method has the advantages of simple and easily available raw materials, relatively mild reaction conditions, wide substrate universality, novel preparation process, less pollution, low energy consumption and the like.
Owner:WENZHOU UNIVERSITY
Mefenpyr-diethyl impurity, preparation method and applications thereof
InactiveCN110724144AHigh economic feasibilityMild reaction conditionsOrganic chemistry methodsTesting foodCarboxylic acidStructural formula
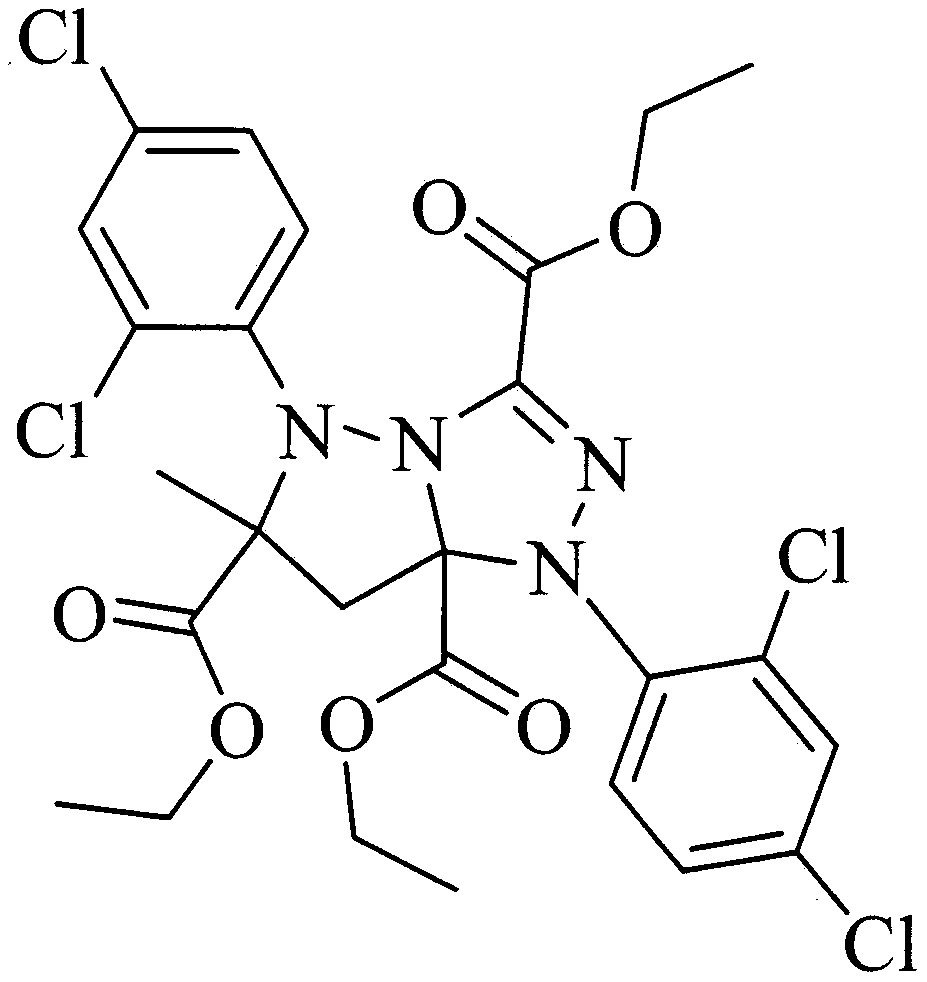
The invention discloses a mefenpyr-diethyl impurity, a preparation method and applications thereof, wherein the molecular structural formula is represented by a formula I. The preparation method comprises: carrying out diazotization and a Japp-Klingemann reaction by using 2,4,-dichloroaniline, nitrite and ethyl 2-chloroacetoacetate as raw materials to obtain an intermediate, and carrying out a ring-closure reaction on the intermediate and ethyl methacrylate to obtain 1,5-bis(2,4-dichlorophenyl)-6-methyl-1H,5H-pyrazolo[5,1-c]-1,2,4-triazole-3,6,7a-triethyl tricarboxylate, wherein the yield reaches more than 40%. According to the invention, the method has advantages of mild reaction conditions, low energy consumption, high economic feasibility and high yield; and the substance has the application value in the influence on the efficacy of mefenpyr-diethyl by using the substance as the impurity and in the safety evaluation of the residual substance on agricultural products.
Owner:江苏艾科姆检测有限公司
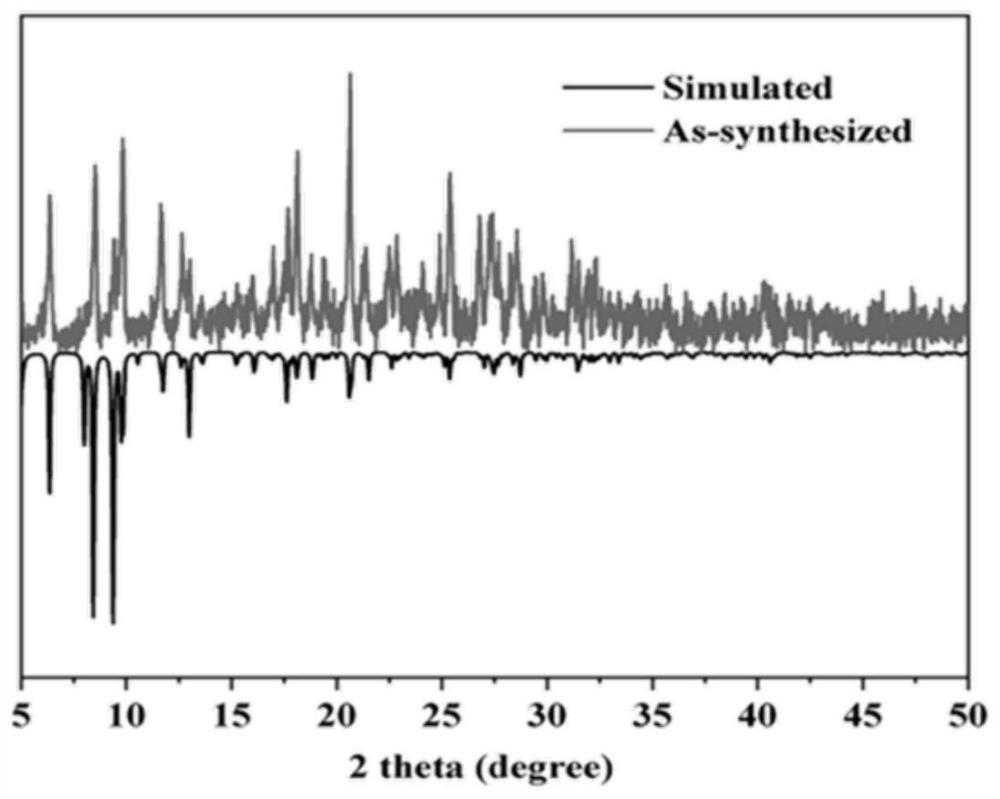
Metal-organic framework material as well as preparation method and application thereof
ActiveCN112876689AImprove adsorption capacityImprove thermal stabilityGroup 1/11 organic compounds without C-metal linkagesOther chemical processesSorbentPhysical chemistry
The invention discloses a metal-organic framework material as well as a preparation method and application thereof. The chemical formula of the metal-organic framework material is [Cu (btr) 2 (hpztr) 2 (pztr<-1>) 2 (NO3<->) 3] n, wherein Hpztr is 4-(3, 5-dimethylpyrazole-4-yl)-1, 2, 4-triazole, and btr is 4, 4'-bis-1, 2, 4-triazole. The preparation method of the material comprises the following steps of: (1) synthesizing Hpztr; (2) synthesizing btr; (3) dissolving copper salt, Hpztr and btr by using a mixed solvent to obtain a mixed solution; and (4) placing the mixed solution in a constant-temperature drying box at 90-100 DEG C for reaction, cooling to room temperature, filtering, washing and drying to obtain the metal-organic framework material. The material can be applied to removal of ions in water. The material has relatively high thermal stability and chemical stability. Anions in water can be effectively adsorbed and enriched at normal temperature and normal pressure, and particularly, the adsorption rate is high and the adsorption capacity is large. The material can be used as an efficient water purifying adsorbent for removing water from water.
Owner:JIANGSU UNIV OF SCI & TECH
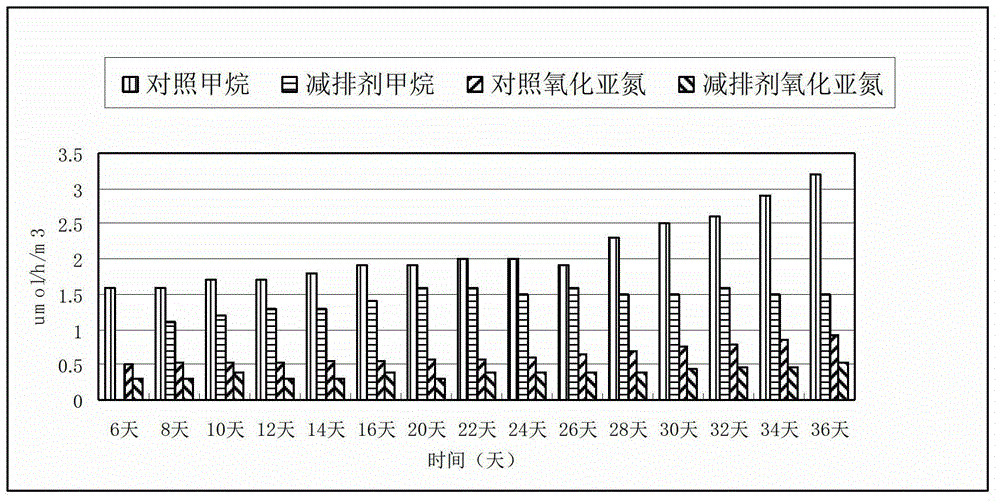
Greenhouse gas emission reduction agent as well as preparation method and application thereof
The invention relates to a greenhouse gas emission reduction agent as well as a preparation method and an application thereof. The greenhouse gas emission reduction agent finished product is obtained through the following steps of: respectively weighing 8-18 parts by weight of dicyandiamide, 10-30 parts by weight of 3,4-dimethyl pyrazol phosphate, 10-20 parts by weight of thiourea-N-2,5-dichlorobenzene succinamide, 10-25 parts by weight of 4-amino-1,2,3-triazole hydrochloride, 0.5-10 parts by weight of alkyl potassium xanthate, 0.5-15 parts by weight of allyl mercaptan and 0.01-0.5 part by weight of monensin; and fully mixing and smashing the materials. The greenhouse gas emission reduction agent disclosed by the invention can be used for effectively inhibiting the activity for producing methane microorganisms, nitrifiers and denitrifying microbes in soil and reducing the production and emission of greenhouse gas (methane and nitrogen monoxide) in the soil, so that the influence of the greenhouse gas on the climate is reduced, environment is protected and the disastrous influence of global climate change on human is reduced.
Owner:吴洪生
Preparation method of nitrogen-phosphorus synergist
PendingCN110078560AIncrease profitReduce usageOrganic phosphatic fertilisersFertilizer mixturesHydrolysateBrassinolide
The invention provides a nitrogen-phosphorus synergist containing loach and mucus hydrolysate thereof, and a preparation method and application of the nitrogen-phosphorus synergist. The nitrogen-phosphorus synergist containing the loach and the mucus hydrolysate thereof comprises the following components in parts by weight: 30-45 parts of the loach and the mucus hydrolysate thereof, 30-60 parts ofblack chokeberry pomace, 5-15 parts of nostoc sphaeroides proteoglycan, 15-30 parts of potassium fulvate, 15-40 parts of polyaspartic acid, 1-5 parts of cyclohexylphosphoric triamide, and 0.5-5 partsof 3,4 dimethylpyrazole, 1-6 parts of sodium alpha-naphthylacetate, 3-9 parts of brassinolide and 2-5 parts of poly-gamma-glutamic acid. The planting test shows that by the nitrogen-phosphorus synergist, the yield of protected vegetables can be increased, use of nitrogen and phosphorus fertilizers is reduced, the contents of available phosphorus and available nitrogen in soil are increased, the quality of the soil can further be improved, and soil-borne diseases are inhibited.
Owner:邓灿辉
Chiral 3-(benzylsulfinyl)-5,5-dimethyl-4,5-dihydroisoxazole derivatives and 5,5-dimethyl-3-[(1H-pyrazol-4-ylmethyl)sulfinyl]-4,5-dihydroisoxazole derivatives, method for the production thereof, and use of same as herbicides and plant growth regulations
InactiveUS8420570B2Improved crop compatibilityImprove herbicidal activityBiocideOrganic chemistryPlant growthChemistry
The invention relates to 3-(benzylsulfinyl)-5,5-dimethyl-4,5-dihydroisoxazole derivatives and 5,5-dimethyl-3-[(1H-pyrazol-4-ylmethyl)sulfinyl]-4,5-dihydroisoxazole derivatives of the formula (I) and their saltsprocesses for their preparation and their use as herbicides and plant growth regulators, in particular as herbicides for the selective control of harmful plants in crops of useful plants.
Owner:BAYER INTELLECTUAL PROPERTY GMBH
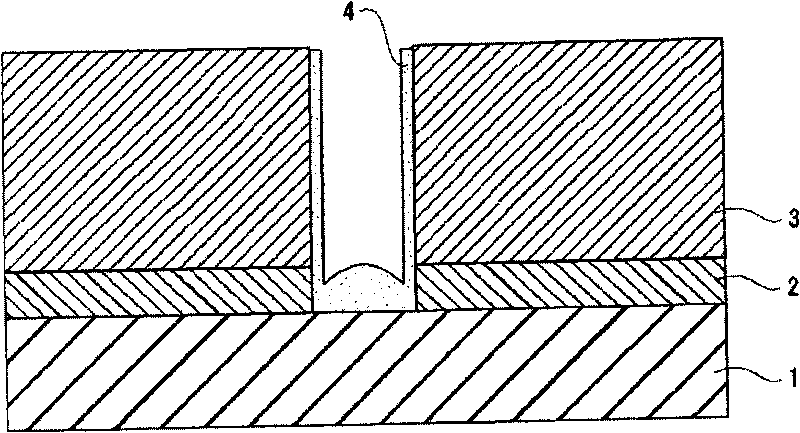
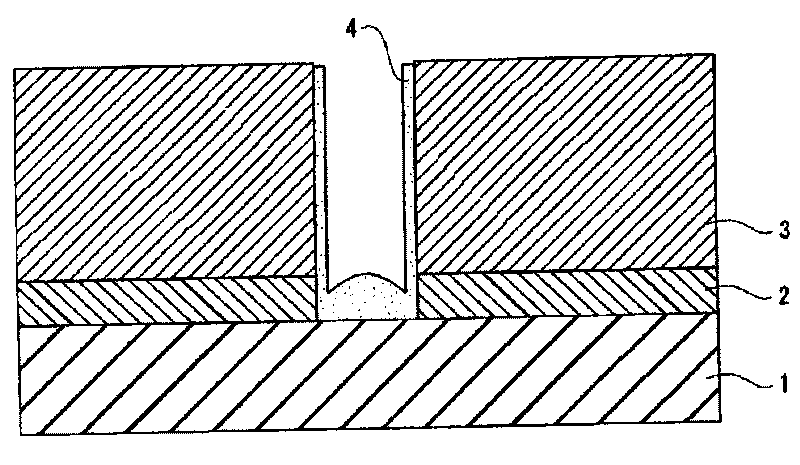
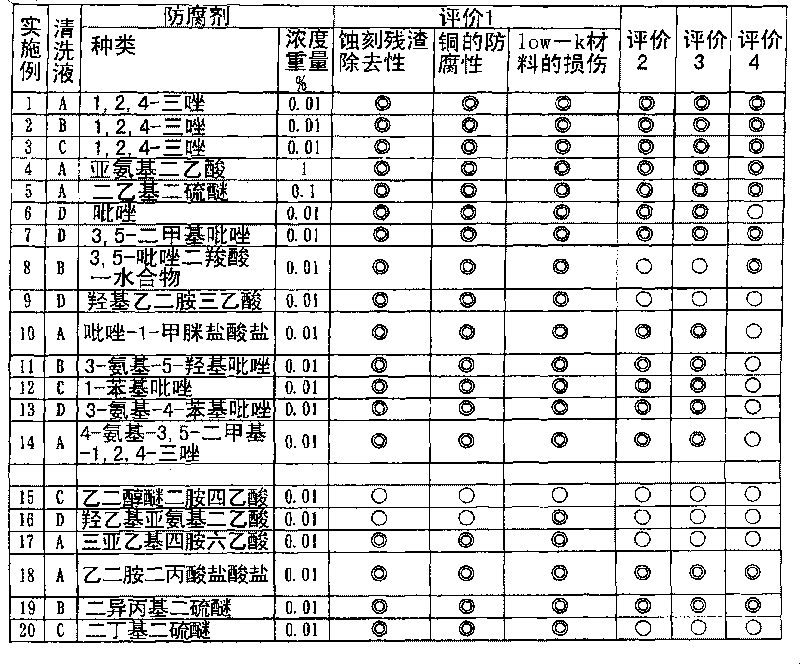
Composition for cleaning and rust prevention and process for producing semiconductor element or display element
ActiveCN101755324AOrganic detergent compounding agentsDetergent mixture composition preparationPropanoic acidEthyl group
A composition for cleaning and rust prevention which is for use in the step of producing, e.g., a semiconductor element having a metallic wiring containing copper. The composition comprises: an anticorrosive component comprising any of pyrazole, pyrazole derivatives such as 3,5-dimethylpyrazole, 1,2,4-triazole, triazole derivatives, aminocarboxylic acid compounds such as iminodiacetic acid and ethylenediaminedipropionic acid hydrochloride, and disulfides such as diisopropyl disulfide and diethyl disulfide; and a detergent component comprising any of ammonium fluoride, tetramethylammonium fluoride, ammonium acetate, acetic acid, glyoxylic acid, oxalic acid, ascorbic acid, 1,2-diaminopropane, and dimethylacetamide. Also provided is a process for producing a semiconductor element or the like using the composition for cleaning and rust prevention.
Owner:MITSUBISHI GAS CHEM CO INC
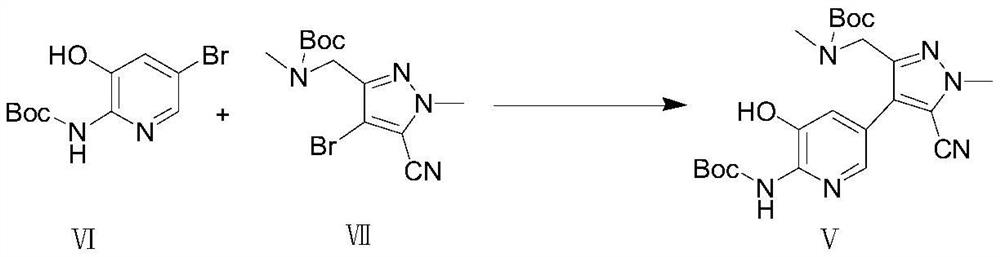
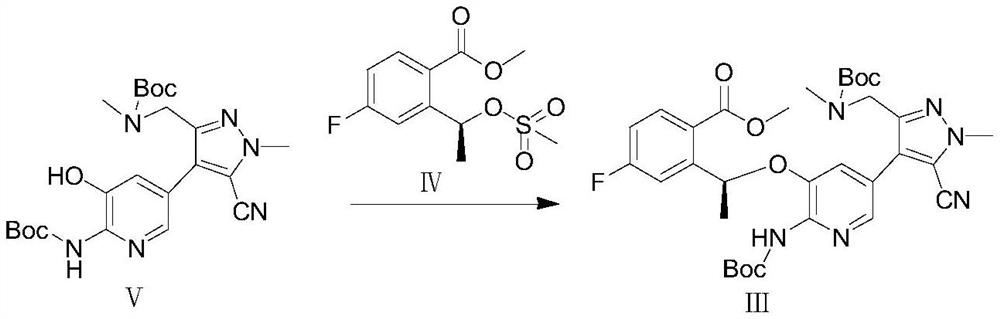
Preparation method of lorlatinib
The invention relates to a preparation method of lorlatinib. Specifically, the invention provides a preparation method of lorlatinib. According to the method, a compound 1-methyl-3-((methyl-t-butyloxycarboryl-amino) methyl)-1H-pyrazole-4-bromine-5-nitrile as shown in a formula VII and a compound 2-(t-butyloxycarboryl-amino)-3-hydroxy-5-bromopyridine as shown in a formula VI are used as raw materials and subjected to a coupling reaction, a Williamson reaction, a hydrolysis reaction, an acidolysis reaction and a condensation reaction, so as to prepare lorlatinib. The preparation method of the lorlatinib has the advantages of being short in synthesis route, simple and convenient to operate, mild in reaction condition, high in yield and the like, and is suitable for industrial production of the lorlatinib.
Owner:SHANGHAI TIANCI INT PHARMA
Reactive acid yellow dye for nylon and preparation method thereof
InactiveCN101575459AImprove bindingImprove wet fastnessReactive dyesDyeing processAnilinePhenyl group
The invention discloses a reactive acid yellow dye for nylon and a preparation method thereof, comprising: cyanuric chloride is reacted with m-phenylenediaminedisulfonic acid by condensation reaction; excessive sodium nitrite is added to perform diazotization; then 15% 1-(2',5'-dicloro-4'-sulfo)phenel-3-methyl-5-pyrazolonem solution is added and mixed evenly, the obtained solution is adjusted by alkali solution to show weak acidic pH and performs couple reaction; finally 20% p-(beta-sulfatoethylsulfonyl)aniline solution is added, appropriate acidity and temperature are adjusted and reactive acid yellow dye is obtained when the temperature is preserved. Not only physical bond but also chemical bonding exists between the reactive acid yellow and nylon fiber, thus showing strong combining capacity. In 30-60 DEG C routine tests, the dyed fabric of invention can be washed for a plurality of times without fading and has good wet-fastness; even on the washing conditions of steaming and detergent, the fabric also shows good fastness performance and migration, bright color and high degree of exhaustion.
Owner:安徽省凤阳染料化工有限公司
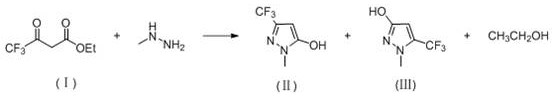
Preparation method of 3-difluoro-methyl pyrazole-4-carboxylic acid and 3-trifluoro-methyl pyrazole-4-carboxylic acid
The invention provides a preparation method of 3-difluoro-methyl-1H-methyl pyrazole-4-carboxylic acid shown in the formula (I) or 3-trifluoro-methyl-1H-methyl pyrazole-4-carboxylic acid shown in the formula (II), and the method comprises the following steps of: (a) providing a 3-polyfluoro methyl-1H-pyrazole-4-nitrile group shown in the formula (VII); and (b) under the condition of participation of acid or alkaline, carrying out hydrolysis reaction on the 3-polyfluoro methyl-1H-pyrazole-4-nitrile group shown in the formula (VII), thus obtaining the 3-difluoro-methyl-1H-methyl pyrazole-4-carboxylic acid shown in the formula (I) or 3-trifluoro-methyl-1H-methyl pyrazole-4-carboxylic acid shown in the formula (II).
Owner:LANZHOU CHEMSPECWEIER CHEM CO LTD
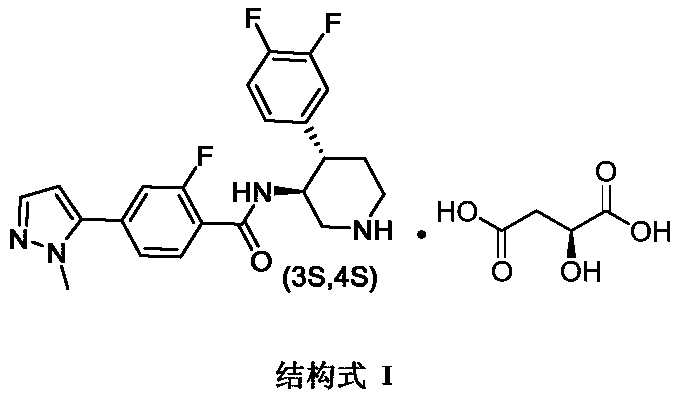
A kind of preparation technology of polyfluorine-substituted aromatic heterocyclic compounds
ActiveCN109574991BEasy to storeOvercome liquidCarboxylic acid salt preparationBulk chemical productionBenzoic acidCarboxylic acid
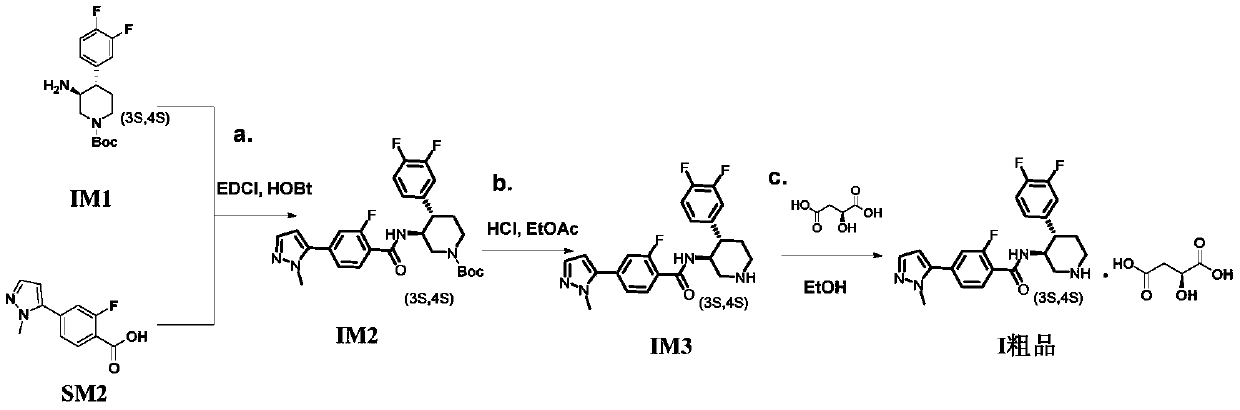
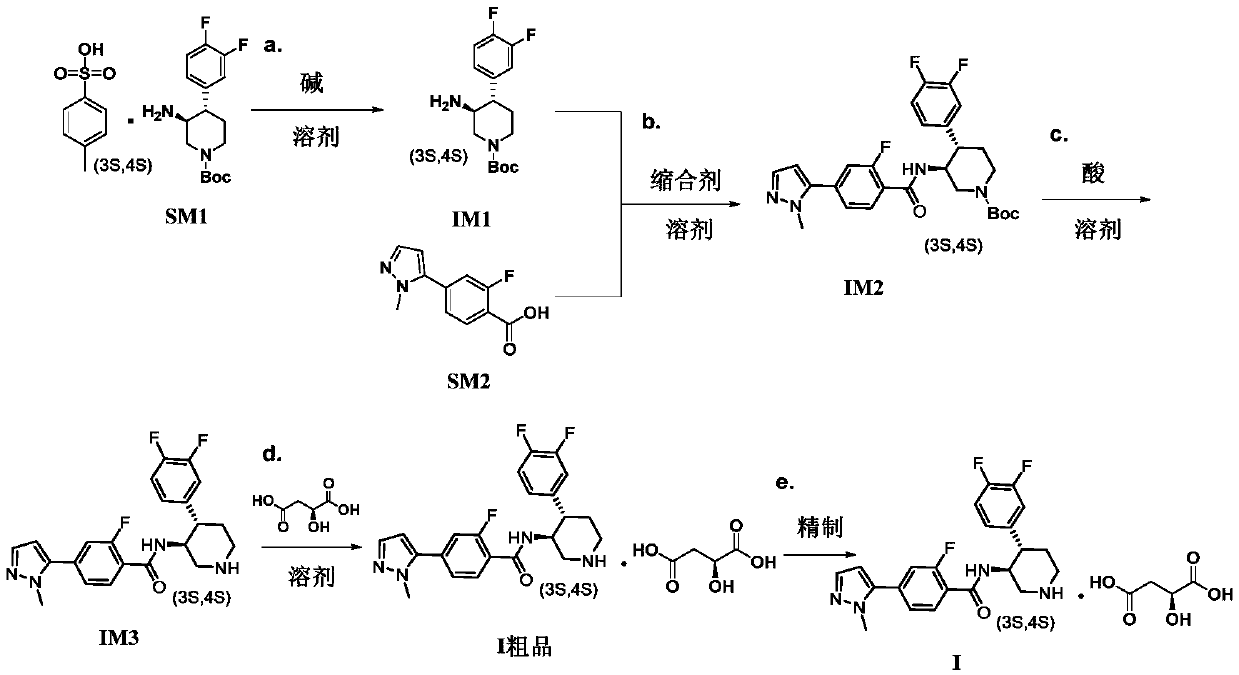
The invention discloses a preparation process of a polyfluoric substituted aromatic heterocycles compound. The preparation process of the polyfluoric substituted aromatic heterocycles compound comprises the steps of taking tert-butyl (3S, 4S)-3-amino-4-(3,4-difluorophenyl)piperidine-1-carboxylate p-toluene sulfonate as a raw material, carrying out dissociation, carrying out condensation reaction on the tert-butyl (3S, 4S)-3-amino-4-(3,4-difluorophenyl)piperidine-1-carboxylate p-toluene sulfonate and 2-fluro-4-(1-methyl-1H-pyrazole-5-yl) benzoic acid, then removing protecting group, and finallysalifying with L-malic acid to obtain 4-(1-methyl-1H-pyrazole-yl)-N-((3S,4S)-4-(3,4-difluorophenyl) piperidine-3-yl)-2-fluorobenzamide L-malate. The used raw material SM1 is solid, the stability is high, weighing and storage are facilitated, the shortcoming that in the prior art, IM1 easily becomes liquid, and is difficult to refine is overcome, an SM1 quality control standard is easily established on production, and raw material suppliers can provide pure SM1; and meanwhile, the chiral purity of the raw material SM1 is high, and the purity and integral yield of the final product are ensuredfrom the source.
Owner:GUANGZHOU LIXIN PHARM CO LTD
Nitrogen stable synergist for irrigation fertilizer and preparation method for nitrogen stable synergist
ActiveCN102649658BInhibit transformationDelay the hydrolysis processFertilizer mixturesThio-Phosphate
Owner:SHENZHEN BATIAN ECOTYPIC ENG +1
Trifluoromethyl pyrazole derivative and applications thereof
PendingCN110627723AEfficient and high-quality large-scale productionEasy to operateOrganic chemistryAntiviralsCycloadditionAlkyne
The invention discloses a trifluoromethyl pyrazole derivative and applications thereof. The trifluoromethyl pyrazole derivative is obtained by utilizing intermolecular trifluorodiazoethane-alkyne cycloaddition reaction catalyzed by Lewis base, can be used for the cycloaddition reaction with terminal / internal alkyne; and a new approach for the synthesis of 3-trifluoromethyl pyrazole is provided. Apreparation method has the characteristics of environmental protection and simple operation, and is suitable for application and large-scale production. The invention also provides applications in preventing and treating cancers, immune system diseases, cardiovascular diseases or infectious diseases.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI +1
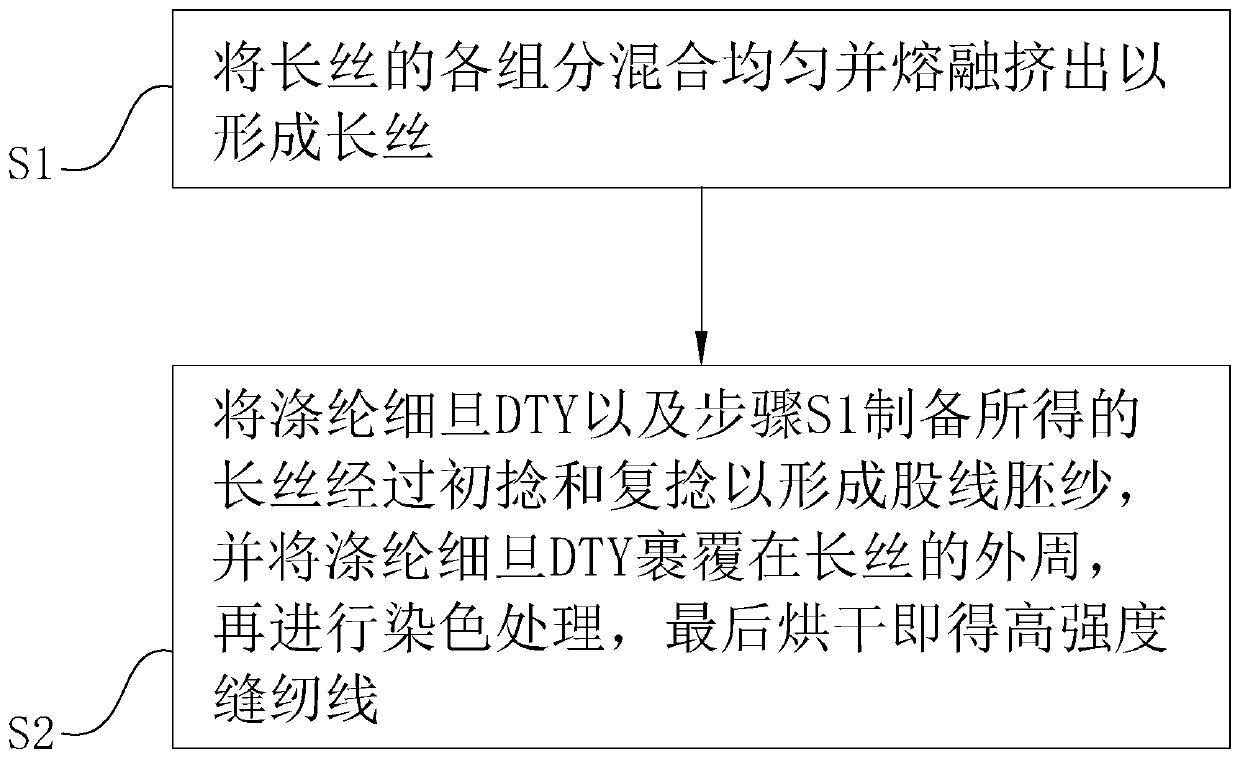
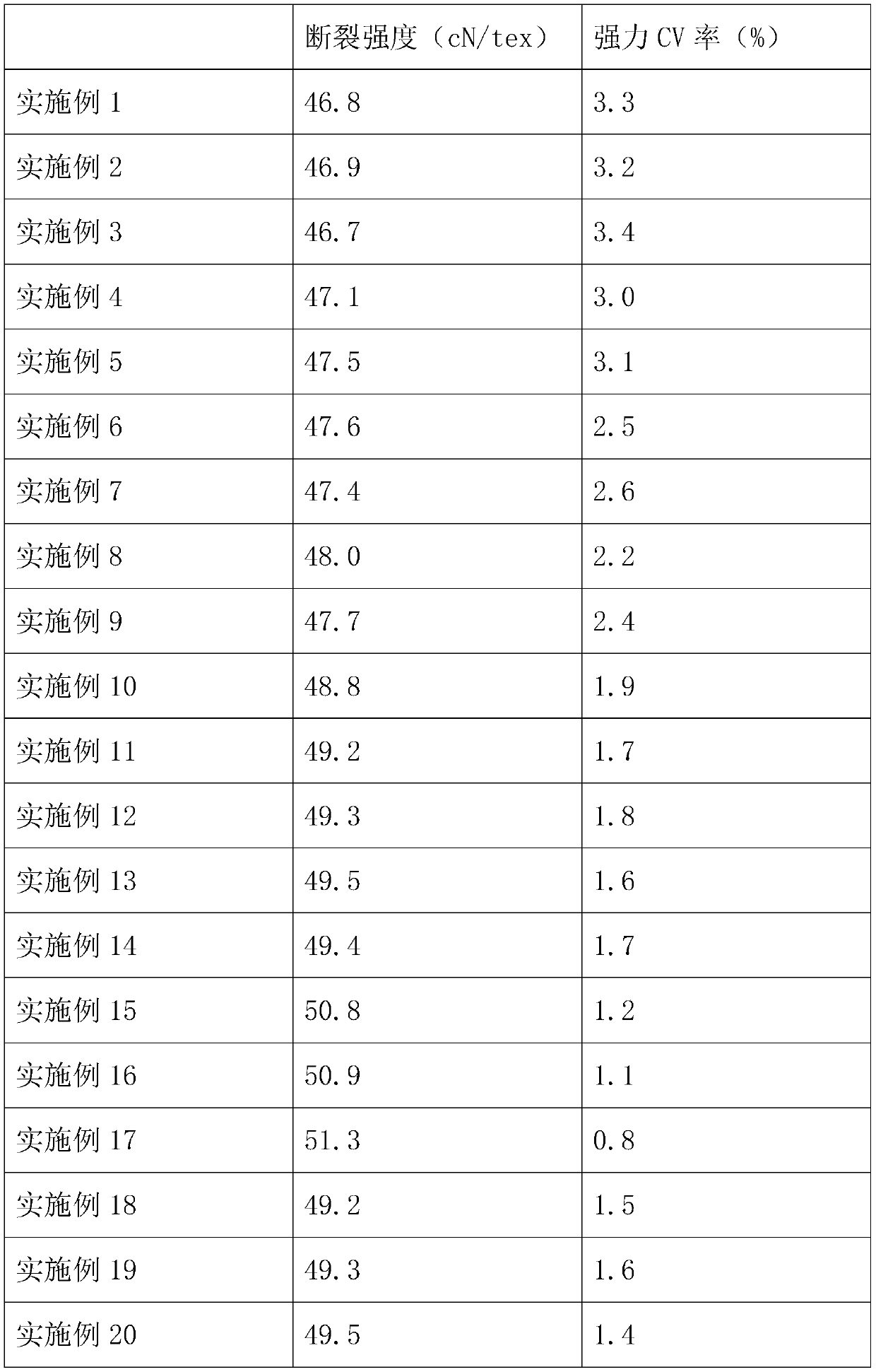
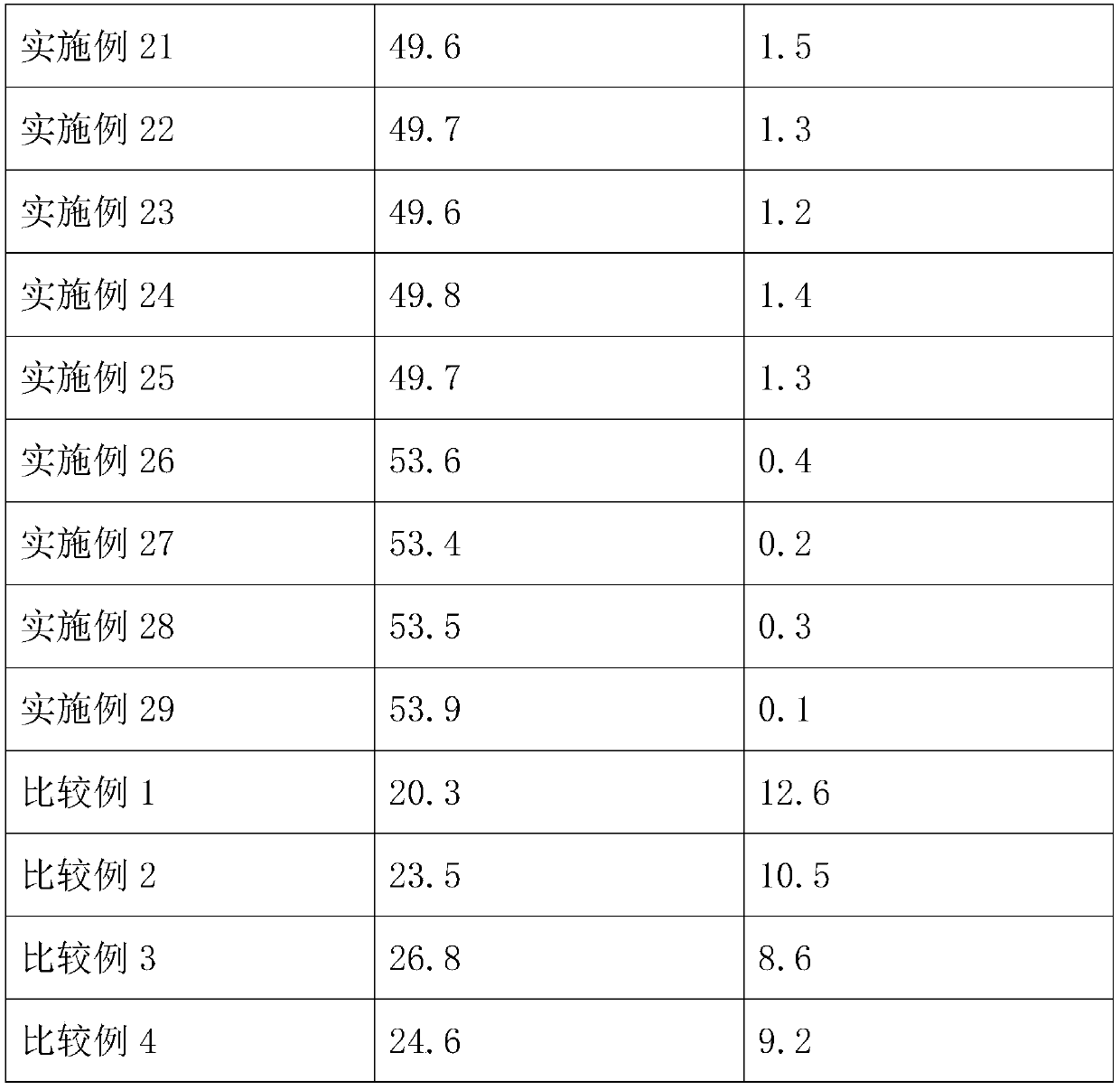
High-strength sewing thread
InactiveCN110735206AHigh breaking strengthReduced strong CV rateConjugated synthetic polymer artificial filamentsYarnYarnPolyester
The invention relates to the technical field of sewing materials, and provides a high-strength sewing thread, aiming at the problem of low strength of existing sewing threads. The high-strength sewingthread comprises a filament and a fine-denier polyester DTY (draw texturing yarn) covering the filament. The filament comprises, by mass, 55-60 parts of polytetrafluoroethylene resin, 25-30 parts ofpolypropylene resin, 1-2 parts of 5-amino-3-cyclohexyl-1-methyl-1H-pyrazole-4-carbonitrile, 0.3-0.5 part of 2-ethyl-4-methyl-6-piperazine-1-pyrimidine and 0.5-1 part of polyoxyethylene glyceryl ether.The high-strength sewing thread has the advantages that through interaction among the 5-amino-3-cyclohexyl-1-methyl-1H-pyrazole-4-carbonitrile, the 2-ethyl-4-methyl-6-piperazine-1-pyrimidine and thepolyoxyethylene glyceryl ether, the breaking strength of the filament is improved, and the strong CV rate of the filament is reduced, so that the strong CV rate of the obtained sewing thread is lowerwhile the breaking strength thereof is higher; the sewing thread easily meets requirements of the society, and the application range of the sewing thread is widened.
Owner:佛山金丝路线业有限公司
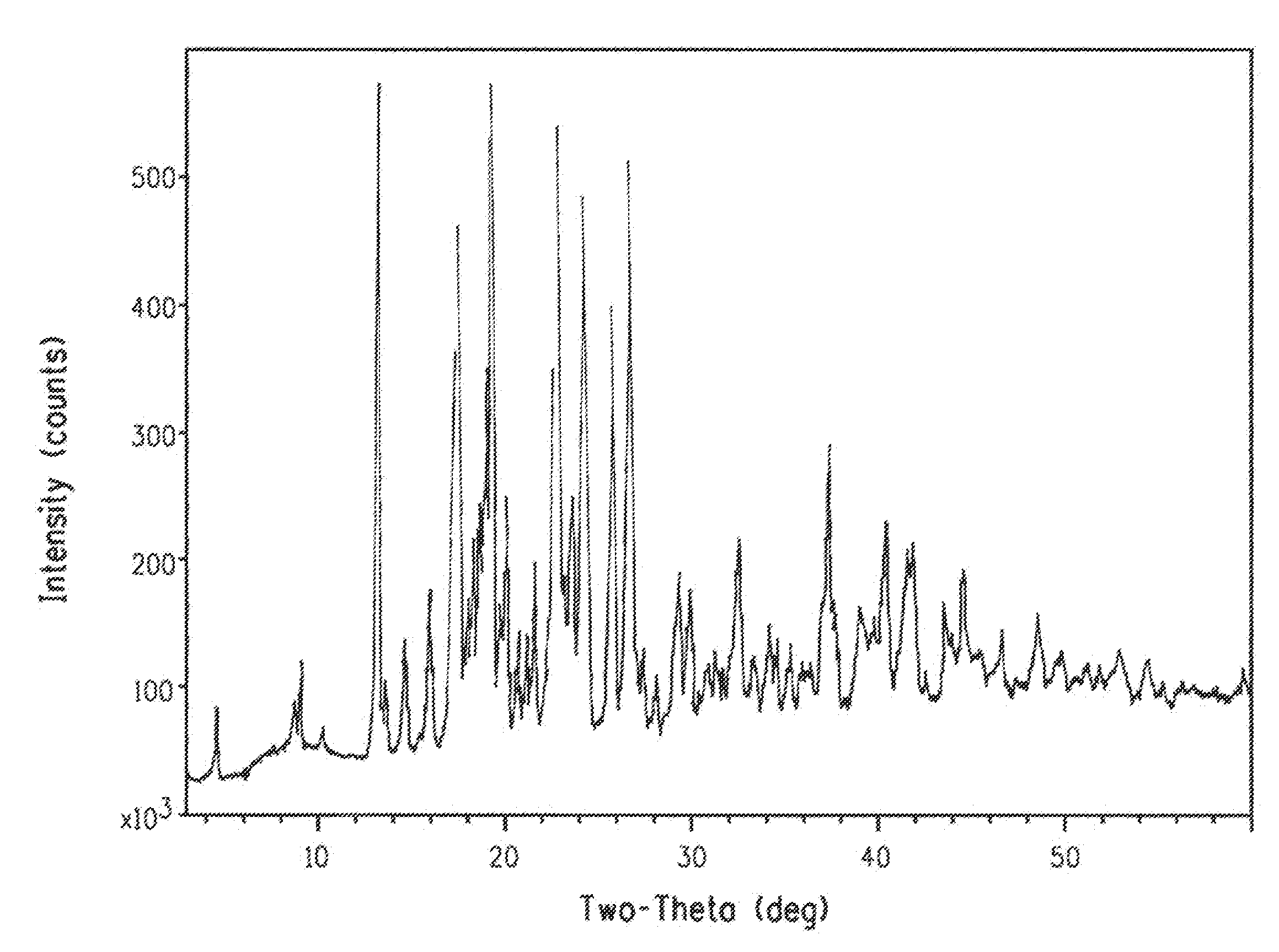
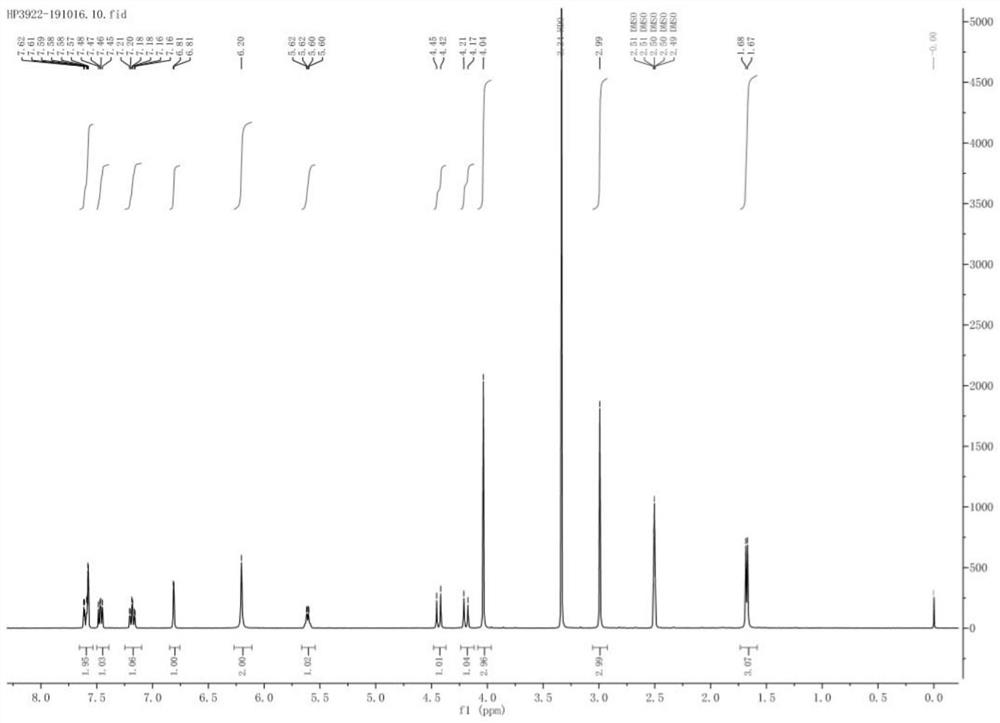
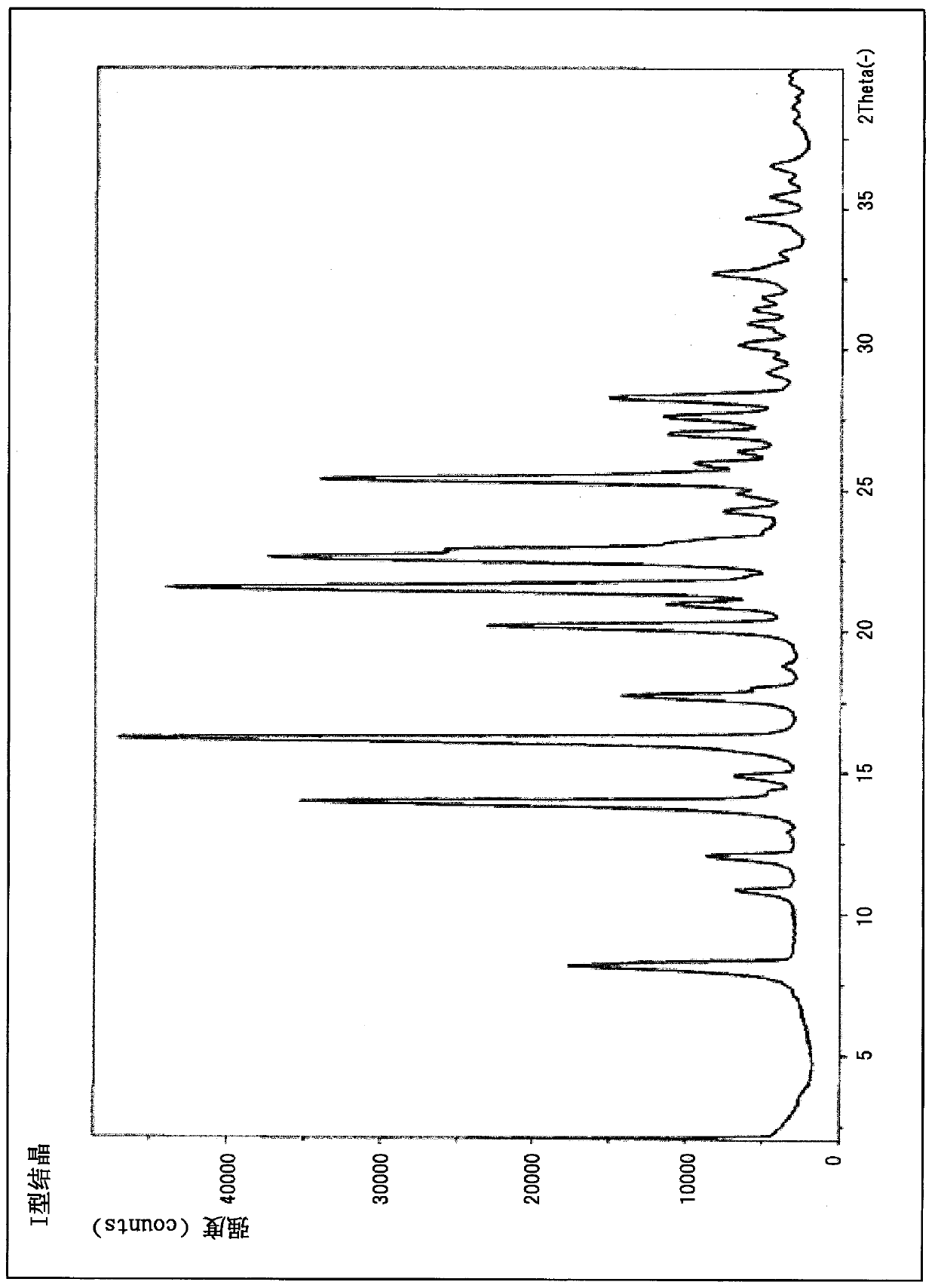
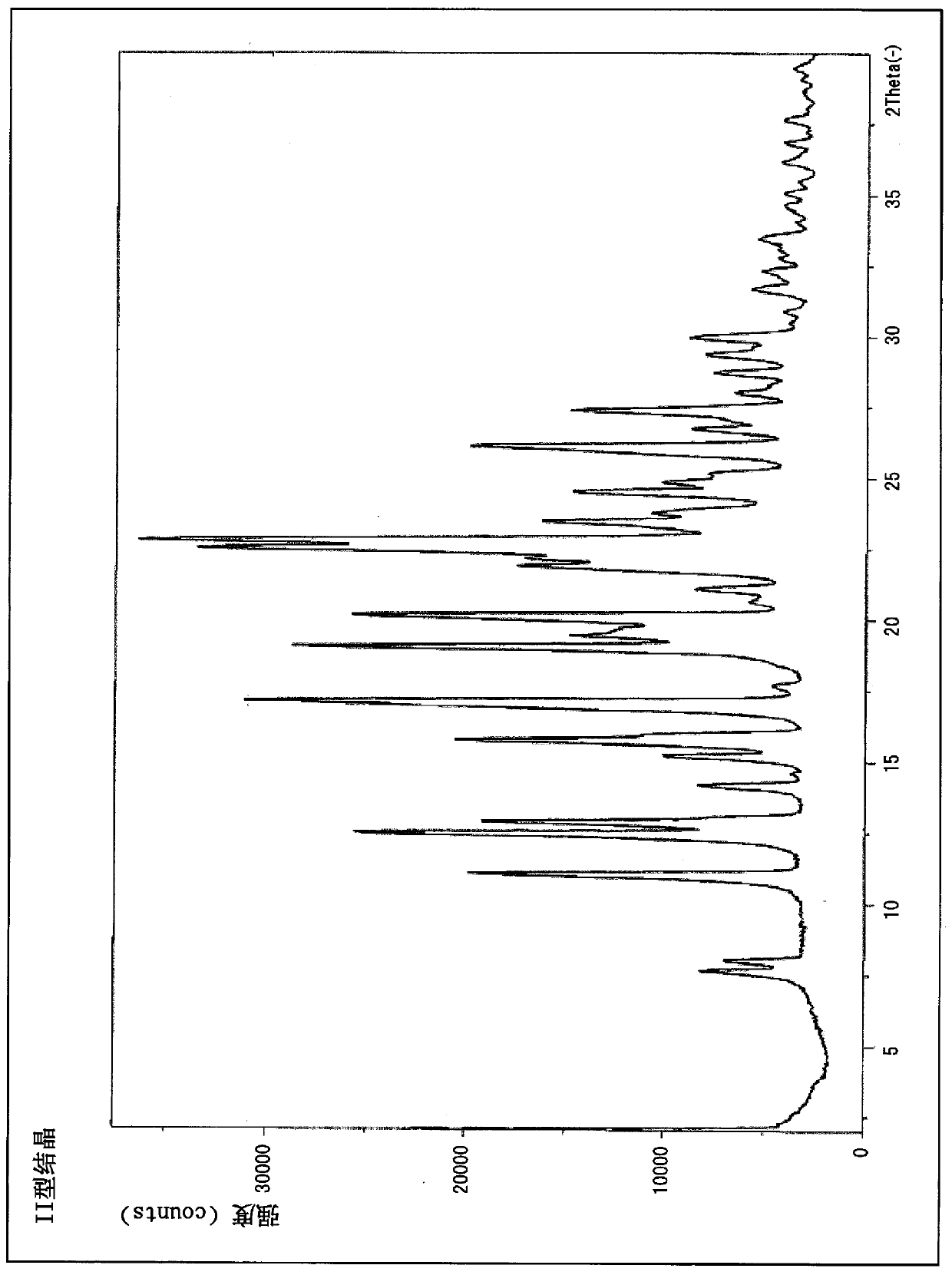
Crystallization of azabicyclic compounds
ActiveCN107531707BImprove stabilityPromote oral absorptionOrganic active ingredientsOrganic chemistry methodsPyrazolylchalconeEthyl group
The invention provides a 3-ethyl-4-{3-isopropyl-4-(4-(1-methyl-1H-pyrazol-4-yl)-1H-imidazol-1-yl)-1H -Pyrazolo[3,4-b]pyridin-1-yl}benzamide is a stable crystal with excellent oral absorbability. 3-ethyl-4-{3-isopropyl-4-(4-(1-methyl-1H-pyrazol-4-yl)-1H-imidazol-1-yl)-1H-pyridine of the present invention Type II crystal of azolo[3,4-b]pyridin-1-yl}benzamide, characterized in that, in the powder X-ray diffraction pattern, it has a diffraction angle (2θ±0.2°) of 7.7°, 8.0° , 11.1°, 12.5°, 12.9°, 15.2°, 15.8°, 17.2°, 19.0°, 22.5°, 26.1° and 27.4° at least three or more characteristic peaks.
Owner:TAIHO PHARMA CO LTD
Synthesis method of high-selectivity 1-methyl-3-(trifluoromethyl)-1H-pyrazole-5-alcohol
ActiveCN113979944AReduce pollutionLow costOrganic chemistryChemical recyclingPtru catalystOrganosolv
The invention discloses a synthesis method of high-selectivity 1-methyl-3-(trifluoromethyl)-1H-pyrazole-5-alcohol, the synthesis method comprises the following steps: taking ethyl 4, 4, 4-trifluoroacetoacetate and a methylhydrazine aqueous solution as raw materials, carrying out condensation cyclization reaction in the presence of an organic solvent and a catalyst, after the reaction is finished, filtering the reaction system, washing and drying, and obtaining the target product 1-methyl-3-(trifluoromethyl)-1H-pyrazole-5-alcohol. According to the invention, cheap raw materials and solvents are adopted, and the reagent ethanol can be generated in the reaction, so that the reaction can be carried out in the forward direction, the reaction efficiency is improved, the reaction conditions are mild, and the reaction process is easy to control; by adding the limited catalyst, the selectivity and the yield of the reaction can be further improved, the yield reaches up to 95% or above, the selectivity of the target product to the byproduct is greater than 99: 1, and the method not only ensures high-selectivity preparation of the target product, but also is relatively high in overall yield, relatively low in cost, less in environmental pollution and suitable for industrial production.
Owner:杭州欧晨科技有限公司
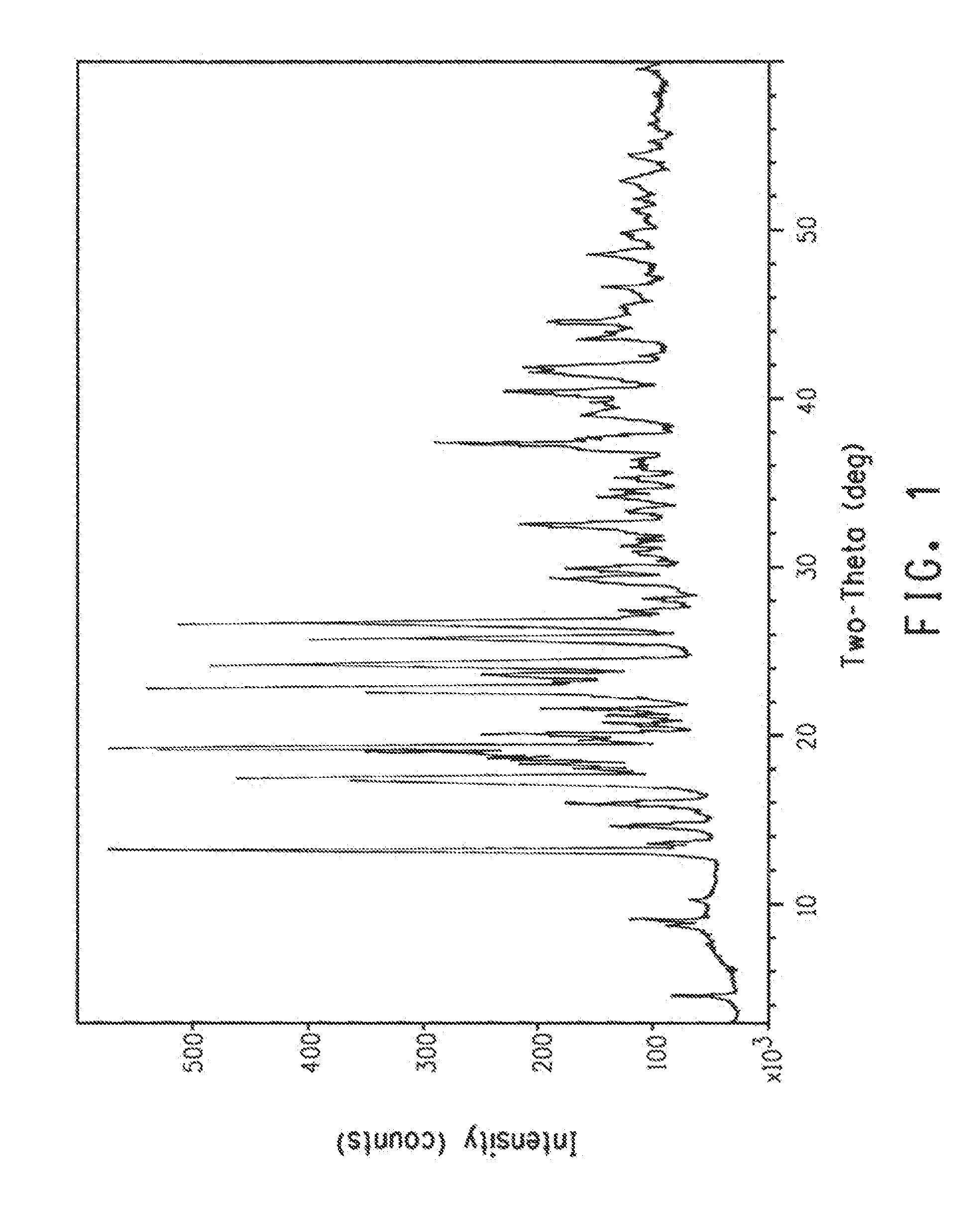
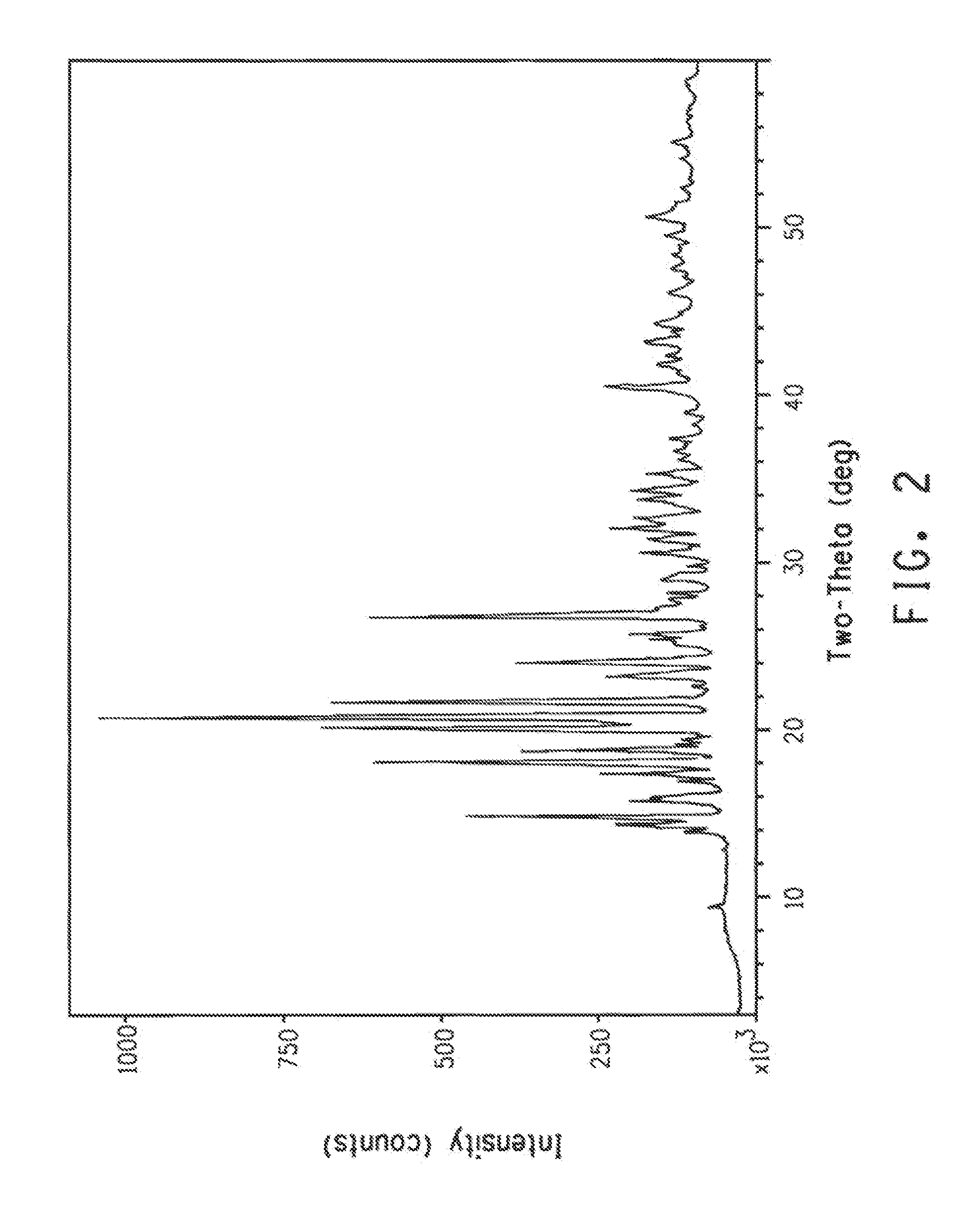
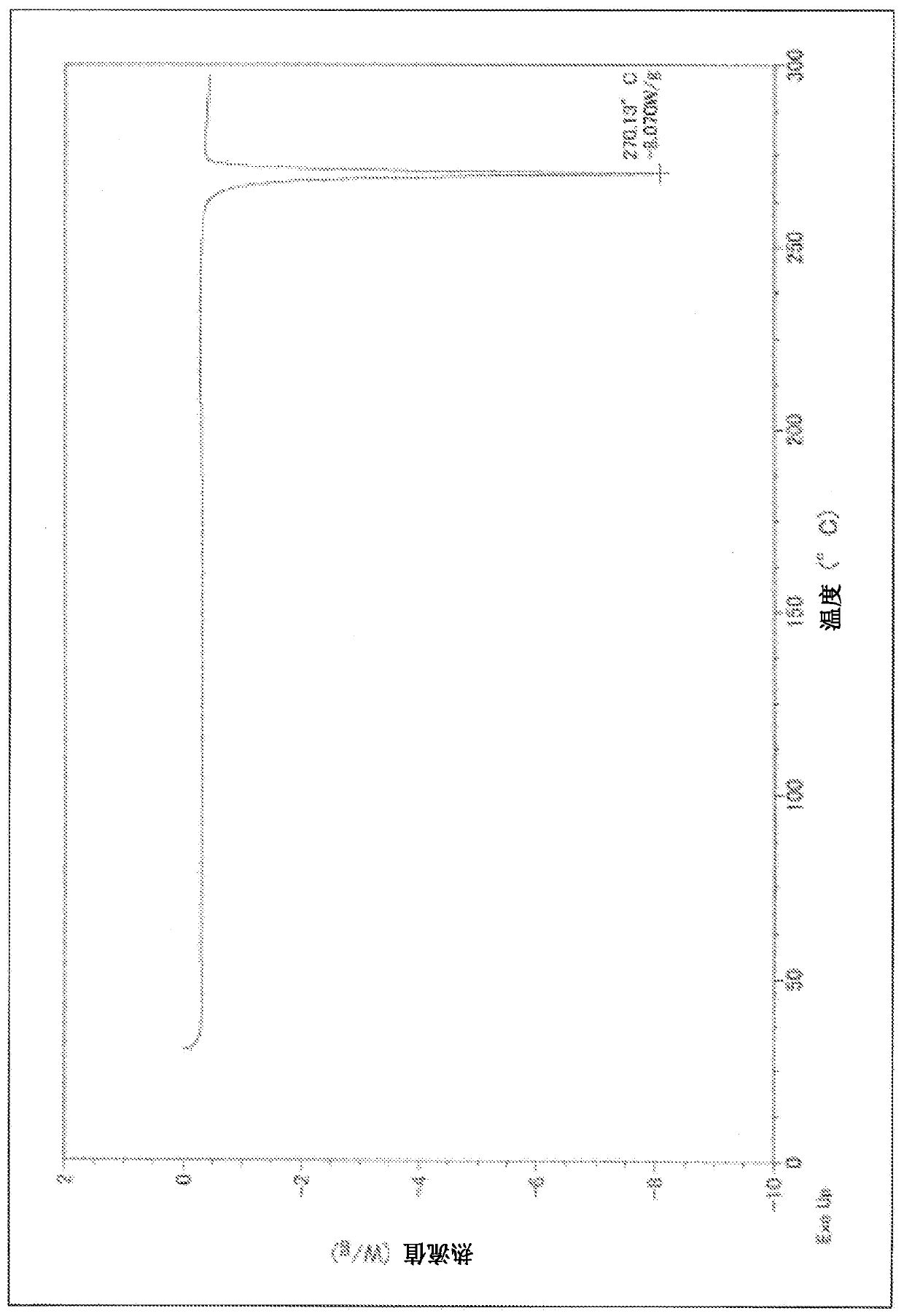
Polymorphic forms of 6-(1-methyl-1H-pyrazol-4-yl)-2-{3-[5-(2-morpholin-4-yl-ethoxy)-pyrimidin-2-yl]-benzyl}-2H-pyridazin-3-one dihydrogenphosphate and processes of manufacturing thereof
ActiveUS8586599B2Increase resistanceHeat stableBiocideOrganic active ingredientsPyridazineMorpholine
The present invention relates to 6-(1-methyl-1H-pyrazol-4-yl)-2-{3-[5-(2-morpholin-4-yl-ethoxy)-pyrimidin-2-yl]-benzyl}-2H-pyridazin-3-one dihydrogenphosphate, its solvates and crystalline modifications thereof. The present invention further relates to processes of manufacturing these crystalline modifications as well as their use in the treatment and / or prophylaxis of physiological and / or pathophysiological conditions, which are caused, mediated and / or propagated by the inhibition, regulation and / or modulation of signal transduction of kinases, in particular by the inhibition of tyrosine kinases, e.g. pathophysiological conditions such as cancer.
Owner:MERCK PATENT GMBH
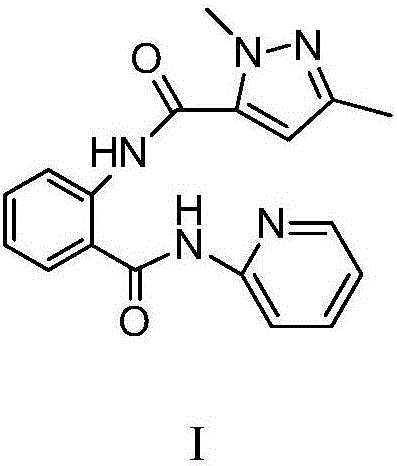
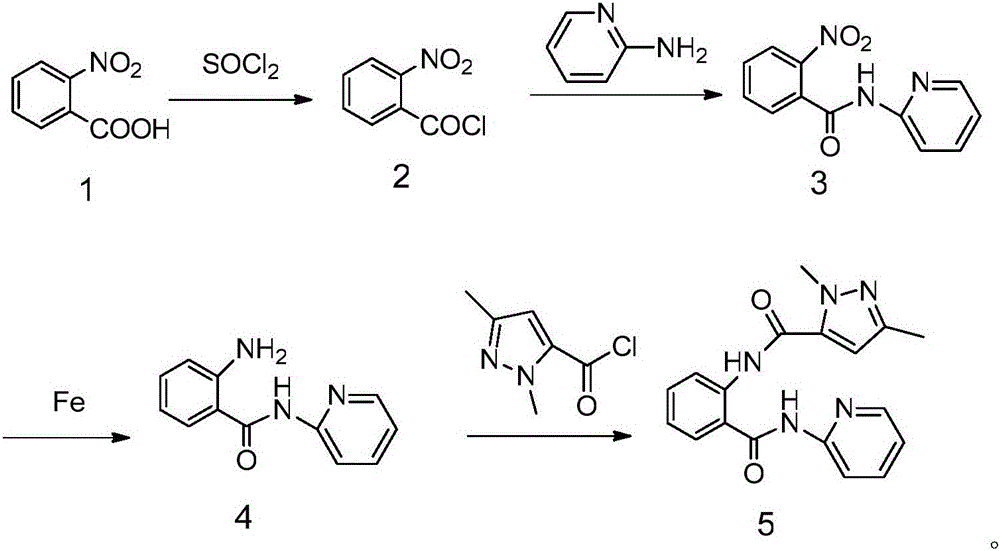
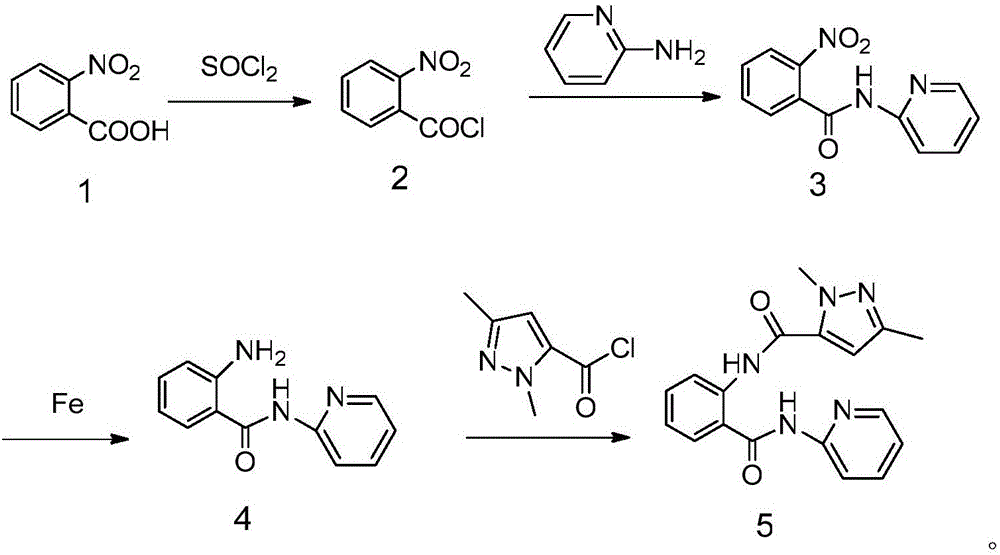
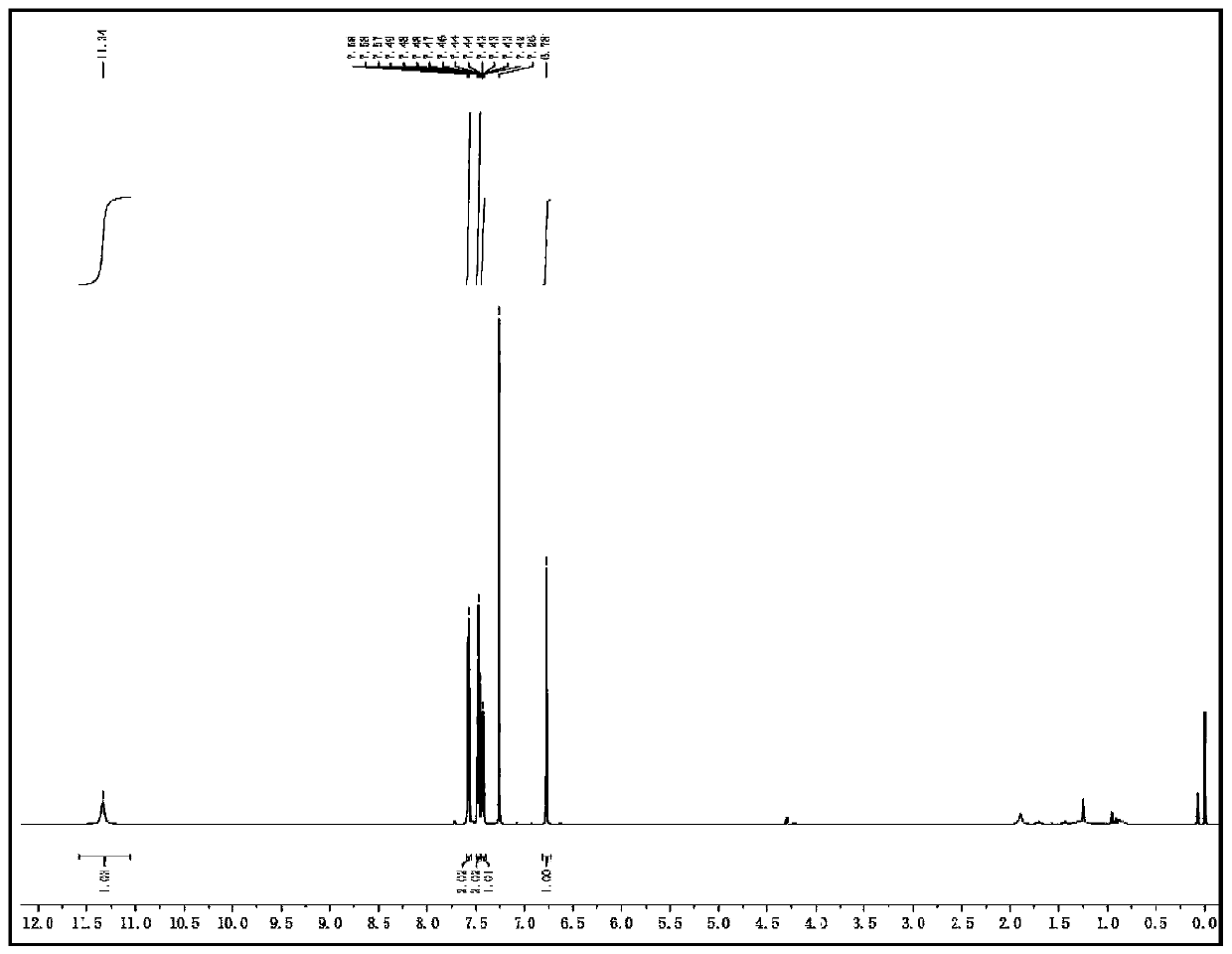
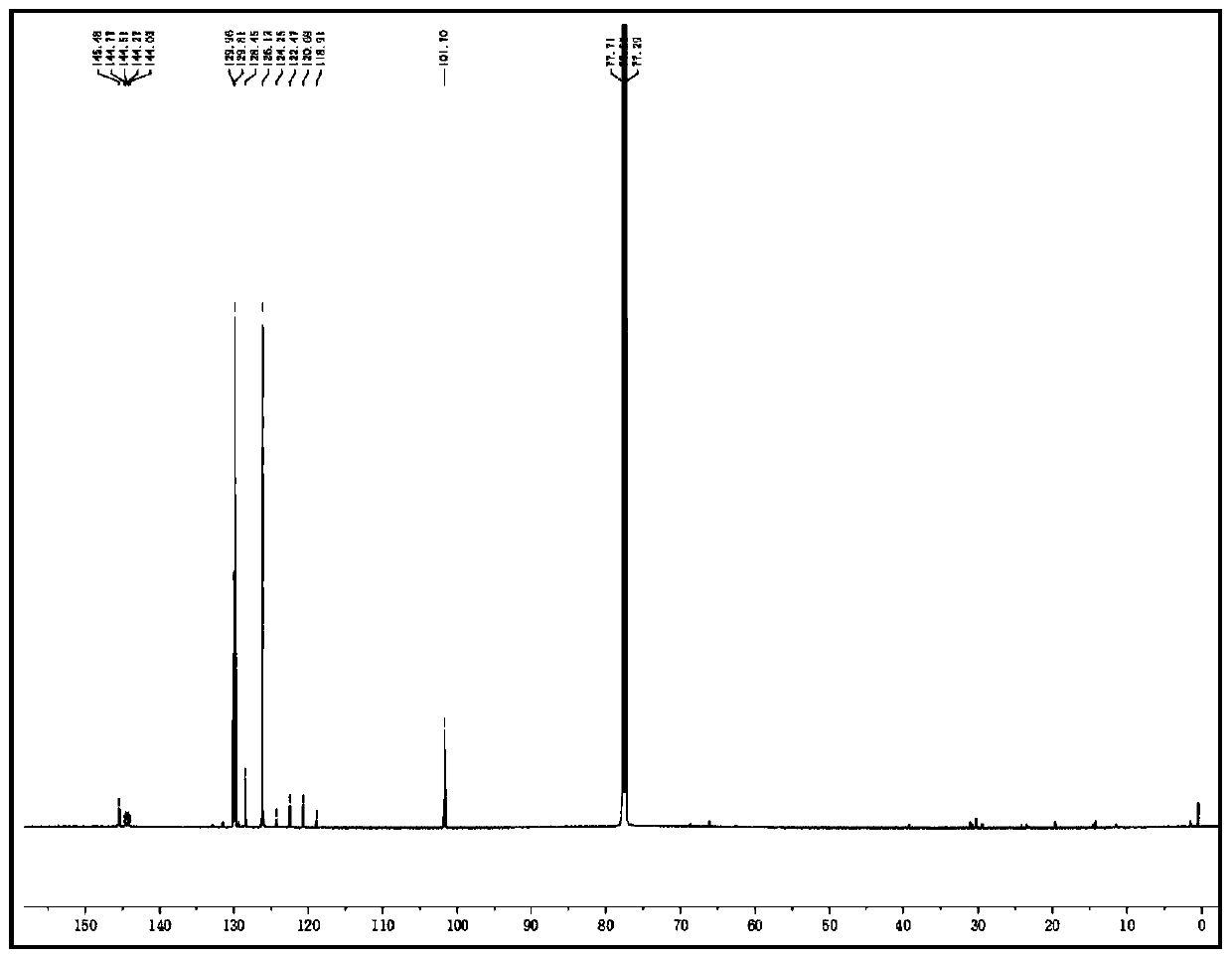
N-(2-pyridyl)-2-(2,4-dimethyl parazole formamido) benzamide as well as preparation thereof and use thereof
InactiveCN105777713AThe synthesis process is simpleHigh antibacterial activityBiocideOrganic chemistryIron powderStructural formula
The invention relates to N-(2-pyridyl)-2-(2,4-dimethyl parazole formamido) benzamide as well as a preparation thereof and use thereof. A structural formula of the N-(2-pyridyl)-2-(2,4-dimethyl parazole formamido) benzamide is as shown in the specification. The preparation method comprises the following steps: taking o-nitrobenzoic acid as a starting material, and carrying out amidation to obtain an intermediate 2-nitrobenzamide; then, reducing nitryl into amido by use of iron powder; and finally, enabling the amido to act with the intermediate acyl chloride under the action of triethylamine, thereby acquiring a target compound N-(2-pyridyl)-2-(2,4-dimethyl parazole formamido) benzamide. The N-(2-pyridyl)-2-(2,4-dimethyl parazole formamido) benzamide has the beneficial effects that the synthesis process is simple; the acquired compound has outstanding bacteriostatic activity on rice sheath blight fungi (Rhizoctorzia solani), and has a bacteriostasis rate of 72.8% on the rice sheath blight fungi under concentration being 1000 ppm.
Owner:WUHAN INSTITUTE OF TECHNOLOGY
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
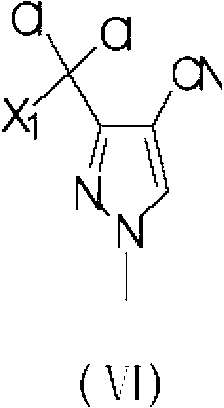
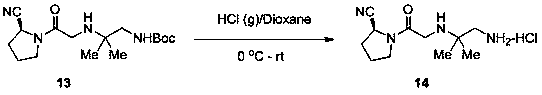


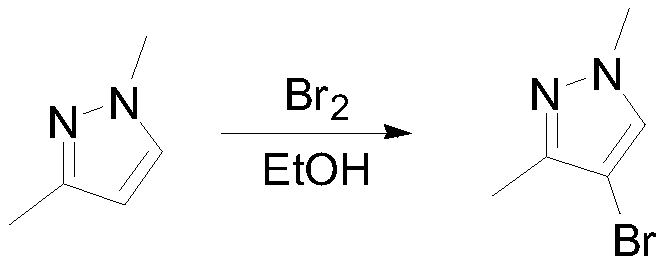


![Chiral 3-(benzylsulfinyl)-5,5-dimethyl-4,5-dihydroisoxazole derivatives and 5,5-dimethyl-3-[(1H-pyrazol-4-ylmethyl)sulfinyl]-4,5-dihydroisoxazole derivatives, method for the production thereof, and use of same as herbicides and plant growth regulations Chiral 3-(benzylsulfinyl)-5,5-dimethyl-4,5-dihydroisoxazole derivatives and 5,5-dimethyl-3-[(1H-pyrazol-4-ylmethyl)sulfinyl]-4,5-dihydroisoxazole derivatives, method for the production thereof, and use of same as herbicides and plant growth regulations](https://images-eureka.patsnap.com/patent_img/6ef1a758-bd09-41f1-adef-e28827c8c31d/US08420570-20130416-C00001.png)
![Chiral 3-(benzylsulfinyl)-5,5-dimethyl-4,5-dihydroisoxazole derivatives and 5,5-dimethyl-3-[(1H-pyrazol-4-ylmethyl)sulfinyl]-4,5-dihydroisoxazole derivatives, method for the production thereof, and use of same as herbicides and plant growth regulations Chiral 3-(benzylsulfinyl)-5,5-dimethyl-4,5-dihydroisoxazole derivatives and 5,5-dimethyl-3-[(1H-pyrazol-4-ylmethyl)sulfinyl]-4,5-dihydroisoxazole derivatives, method for the production thereof, and use of same as herbicides and plant growth regulations](https://images-eureka.patsnap.com/patent_img/6ef1a758-bd09-41f1-adef-e28827c8c31d/US08420570-20130416-C00002.png)
![Chiral 3-(benzylsulfinyl)-5,5-dimethyl-4,5-dihydroisoxazole derivatives and 5,5-dimethyl-3-[(1H-pyrazol-4-ylmethyl)sulfinyl]-4,5-dihydroisoxazole derivatives, method for the production thereof, and use of same as herbicides and plant growth regulations Chiral 3-(benzylsulfinyl)-5,5-dimethyl-4,5-dihydroisoxazole derivatives and 5,5-dimethyl-3-[(1H-pyrazol-4-ylmethyl)sulfinyl]-4,5-dihydroisoxazole derivatives, method for the production thereof, and use of same as herbicides and plant growth regulations](https://images-eureka.patsnap.com/patent_img/6ef1a758-bd09-41f1-adef-e28827c8c31d/US08420570-20130416-C00003.png)

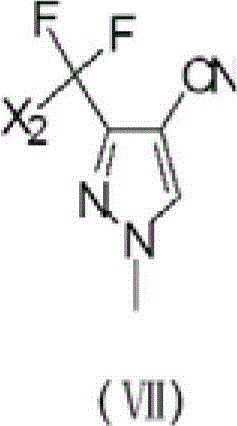
![Polymorphic forms of 6-(1-methyl-1H-pyrazol-4-yl)-2-{3-[5-(2-morpholin-4-yl-ethoxy)-pyrimidin-2-yl]-benzyl}-2H-pyridazin-3-one dihydrogenphosphate and processes of manufacturing thereof Polymorphic forms of 6-(1-methyl-1H-pyrazol-4-yl)-2-{3-[5-(2-morpholin-4-yl-ethoxy)-pyrimidin-2-yl]-benzyl}-2H-pyridazin-3-one dihydrogenphosphate and processes of manufacturing thereof](https://images-eureka.patsnap.com/patent_img/be545bd2-7dec-4bf6-8645-1c2739b05a7c/US08586599-20131119-D00001.png)
![Polymorphic forms of 6-(1-methyl-1H-pyrazol-4-yl)-2-{3-[5-(2-morpholin-4-yl-ethoxy)-pyrimidin-2-yl]-benzyl}-2H-pyridazin-3-one dihydrogenphosphate and processes of manufacturing thereof Polymorphic forms of 6-(1-methyl-1H-pyrazol-4-yl)-2-{3-[5-(2-morpholin-4-yl-ethoxy)-pyrimidin-2-yl]-benzyl}-2H-pyridazin-3-one dihydrogenphosphate and processes of manufacturing thereof](https://images-eureka.patsnap.com/patent_img/be545bd2-7dec-4bf6-8645-1c2739b05a7c/US08586599-20131119-D00002.png)
![Polymorphic forms of 6-(1-methyl-1H-pyrazol-4-yl)-2-{3-[5-(2-morpholin-4-yl-ethoxy)-pyrimidin-2-yl]-benzyl}-2H-pyridazin-3-one dihydrogenphosphate and processes of manufacturing thereof Polymorphic forms of 6-(1-methyl-1H-pyrazol-4-yl)-2-{3-[5-(2-morpholin-4-yl-ethoxy)-pyrimidin-2-yl]-benzyl}-2H-pyridazin-3-one dihydrogenphosphate and processes of manufacturing thereof](https://images-eureka.patsnap.com/patent_img/be545bd2-7dec-4bf6-8645-1c2739b05a7c/US08586599-20131119-D00003.png)