Crystallization of azabicyclic compounds
A technology of crystallization and pharmaceutical composition, applied in the field of crystallization, to achieve excellent oral absorbability and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0062] Hereinafter, the present invention will be described more specifically with reference to examples, but the present invention is not limited to these examples at all. Although the present invention has been described in detail using examples, it will be understood by those skilled in the art that various changes and modifications can be made. Therefore, as long as such changes or modifications do not depart from the scope of the present invention, they are also included in the scope of the present invention.
[0063] Unless otherwise specified, various reagents used in the examples were commercially available. The NMR spectrum uses AL400 (400MHz, Japan Electronics (JEOL)), Mercury400 (400MHz, Agilent Thechnologies) spectrometer, or an Inova 400 (400MHz, Agilent Thechnologies) spectrometer equipped with a 400M NMR probe (Protasis), in a deuterated solvent When tetramethylsilane is contained, measurement is performed using tetramethylsilane as an internal standard, and ot...
Embodiment 1
[0104] Example 1 3-ethyl-4-{3-isopropyl-4-(4-(1-methyl-1H-pyrazol-4-yl)- Synthesis of Type II Crystal of 1H-Imidazol-1-yl)-1H-Pyrazolo[3,4-b]pyridin-1-yl}benzamide
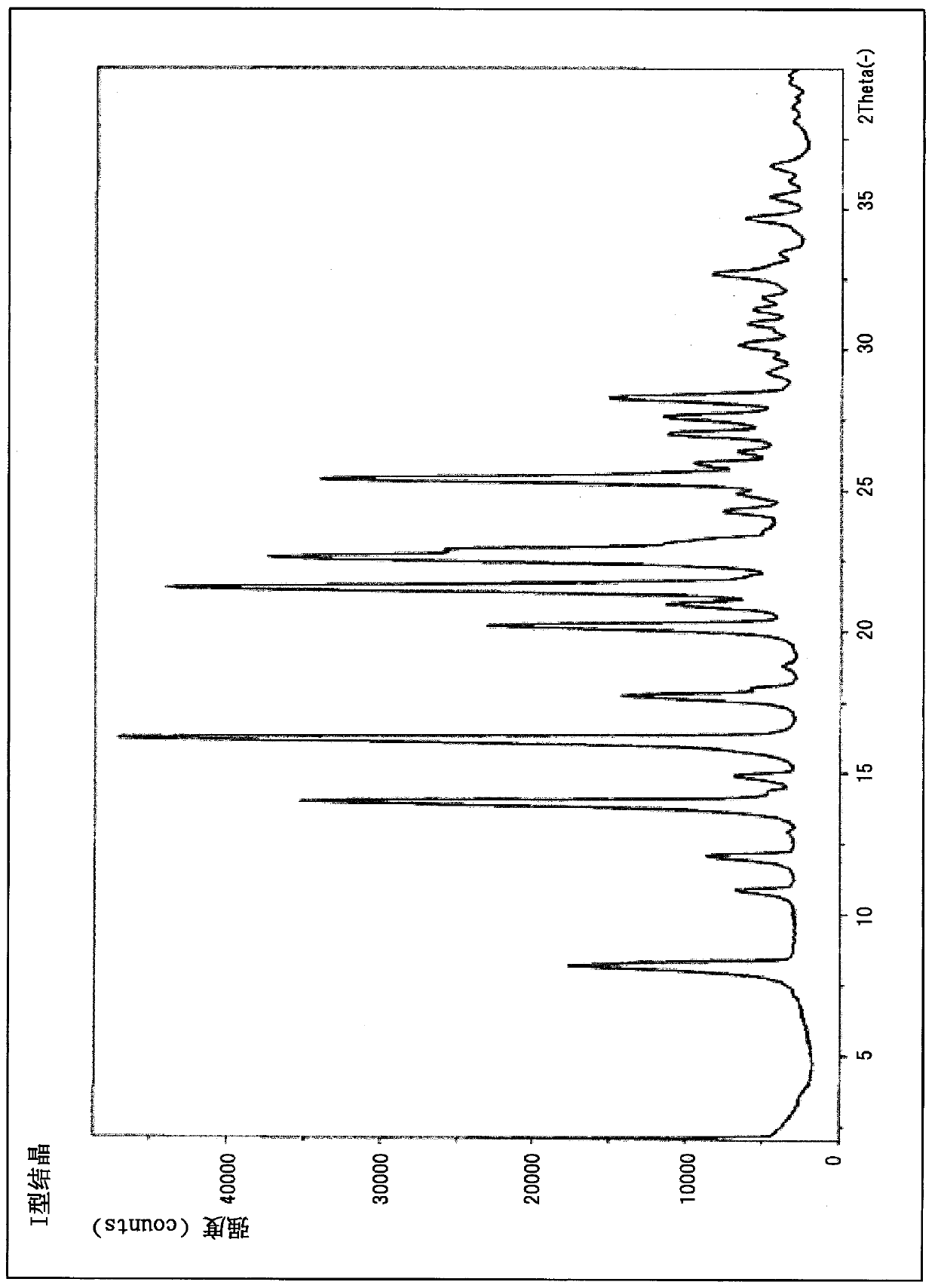
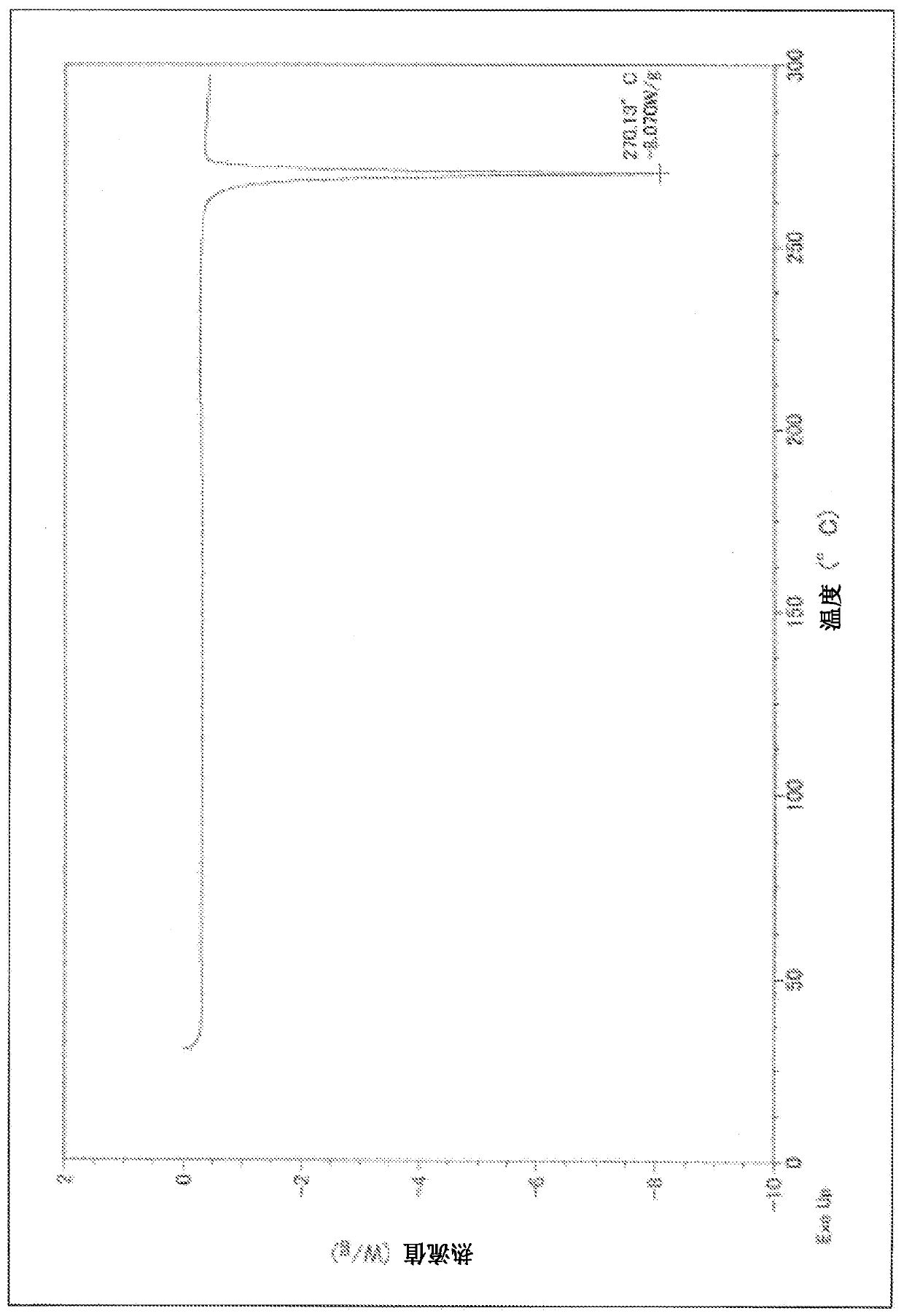
[0105] 3-Ethyl-4-{3-isopropyl-4-(4-(1-methyl Base-1H-pyrazol-4-yl)-1H-imidazol-1-yl)-1H-pyrazolo[3,4-b]pyridin-1-yl}benzamide as a white solid (4.0 g) was added into acetone (19.54 mL), and stirred under reflux for 16 hours. After cooling to room temperature, the solid was collected by filtration, washed with acetone (8.4 mL), and then dried under reduced pressure at 70-80° C. for 16-24 hours to obtain type II crystals (yield: 1.59 g, yield: 57.0%, purity 98.37%).
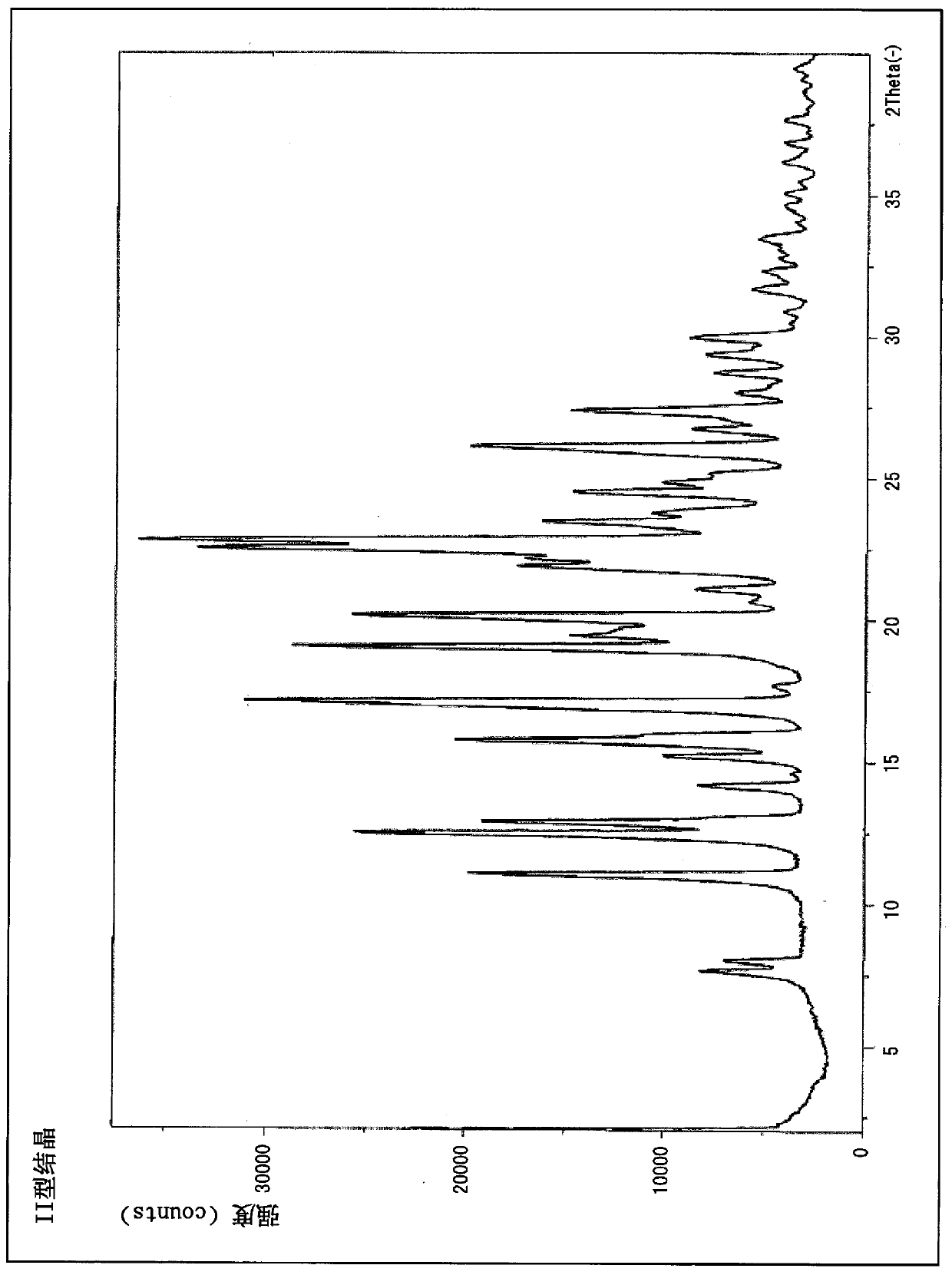
[0106] In addition, type II crystals such as figure 2 Diffraction angles (2θ) 7.7°, 8.0°, 11.1°, 12.5°, 12.9°, 14.2°, 15.2°, 15.8°, 17.2°, 17.7°, 19.0°, 20.2° are shown in the powder X-ray diffraction pattern , 21.1°, 22.5°, 22.8°, 23.5°, 24.5°, 26.1°, 26.7°, 27.4°, 28.0°, 28.7°, 29.4°, 30.0°, 31.7°, 35.1°, 36.2°, 36.9°, and 37.6 ° characteri...
Embodiment 2
[0108] Example 2 3-ethyl-4-{3-isopropyl-4-(4-(1-methyl-1H-pyrazol-4-yl)- Synthesis of Type II Crystal of 1H-Imidazol-1-yl)-1H-Pyrazolo[3,4-b]pyridin-1-yl}benzamide
[0109] 3-Ethyl-4-{3-isopropyl-4-(4-(1-methyl A white solid (400 mg) of 1-1H-pyrazol-4-yl)-1H-imidazol-1-yl)-1H-pyrazolo[3,4-b]pyridin-1-yl}benzamide was added to methyl ethyl ketone (2.8 mL), and stirred under reflux for 16 hours. After cooling to room temperature, the solid was collected by filtration, washed with methyl ethyl ketone (1.2 mL), and then dried under reduced pressure at 70-80°C for 16-24 hours to obtain type II crystals (yield: 197 mg, yield: 60.9%, Purity 98.83%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| width | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com