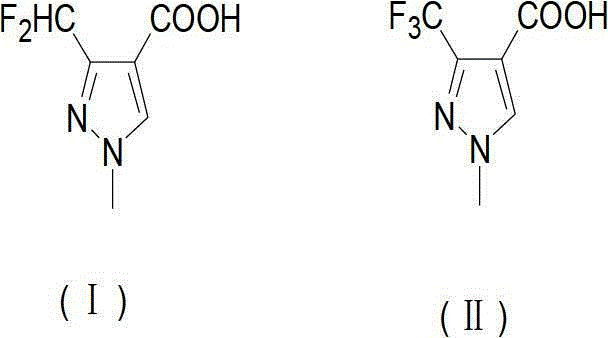
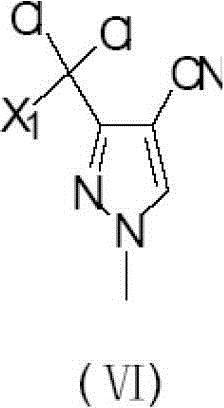
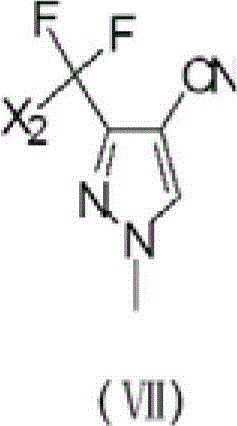Preparation method of 3-difluoro-methyl pyrazole-4-carboxylic acid and 3-trifluoro-methyl pyrazole-4-carboxylic acid
A technology of methylpyrazole and difluoromethyl is applied in the field of preparation of 3-difluoromethylpyrazole-4-carboxylic acid and 3-trifluoromethylpyrazole-4-carboxylic acid, and can solve the problem of reaction Harsh equipment requirements, poor selectivity, waste of fluorine sources, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0141] The present invention provides a preferred embodiment, the method for preparing 3-difluoromethyl-1H-methylpyrazole-4-carboxylic acid and 3-trifluoromethyl-1H-methylpyrazole-4-carboxylic acid comprises the following Steps: one is the reaction of acid chloride (Ⅲ) and compound 3-amino-acrylonitrile (Ⅳ) under the action of a base to generate compound 2-polyhaloacyl-3-amino-acrylonitrile (Ⅴ); the other is compound 2- Polyhaloacyl-3-amino-acrylonitrile (Ⅴ) reacts with methylhydrazine to generate compound 3-polychloromethyl-1H-pyrazole-4-nitrile (Ⅵ); the third is compound 3-polychloro Substituted methyl-1H-pyrazole-4-nitrile (VI) reacts with HF to generate compound 3-polyfluoromethyl-1H-pyrazole-4-nitrile (VII); the fourth is compound 3-polyfluoro Substituted methyl-1H-pyrazole-4-carbonitrile (VII) is hydrolyzed in an aqueous solution of inorganic acid or alkali to prepare 3-difluoromethyl-1H-methylpyrazole-4-carboxylic acid (I) or 3-trifluoromethyl-1H-methylpyrazole-4-carbo...
Embodiment 1-1
[0194] Preparation of 2-dichloroacetyl-3-dimethylamino-acrylonitrile
[0195] Dissolve 26g of 3-dimethylaminoacrylonitrile in 150ml of toluene, add 30.2g of 2,6-lutidine, cool to an internal temperature of 0-10°C, start to add 47.5g of dichloroacetyl chloride dropwise for about one hour Finish. After the dropwise addition, react for another 2 hours. After the reaction is completed, add water, separate the liquid, and concentrate the organic phase to obtain 56 g of the product 2-dichloroacetyl-3-dimethylamino-acrylonitrile. The GC purity is 98.8%, and the yield is 97.0%. .
Embodiment 1-2
[0197] Preparation of 2-dichloroacetyl-3-diethylamino-acrylonitrile
[0198] Dissolve 34g of 3-diethylaminoacrylonitrile in 150ml of chlorobenzene, add 33.2g of 2-methyl-5-ethylpyridine, cool to an internal temperature of -20-0°C, and start to add 47.5g of dichloroacetyl chloride dropwise, about The dropwise addition was completed in one hour. After the dropwise addition, react for another 2 hours. After the reaction is completed, add water, separate the liquid, and concentrate the organic phase to obtain 56 g of the product 2-dichloroacetyl-3-diethylamino-acrylonitrile, with a GC purity of 99.6% and a yield of 95.0%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com