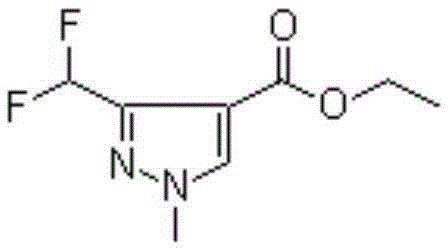
Synthesis method of 3-difluoromethyl-1-methylpyrazole-4-formic acid
A technology of methylpyrazole and difluoromethyl, which is applied in the field of synthesis of difluoromethylpyrazole compounds, can solve the problems of unobtainable raw materials, low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
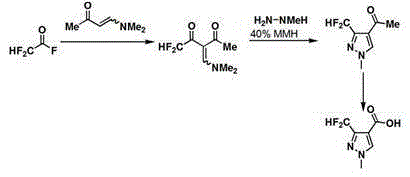
[0050] 102ml of 0.87mol 1-dimethylamino-1-buten-3-one was added to a solution of 500ml of dichloromethane and 254ml of triethylamine, the mixture was cooled to -10°C, and 86 ml of 1.29 mol of difluoroacetyl fluoride. After the addition was complete, the temperature of the reactants was kept at 0°C, and the mixture was continued to react for half an hour. Extract with 500ml of water, then extract the organic matter in the water with 500ml of dichloromethane, combine the organic phases, distill off the dichloromethane organic solvent under reduced pressure, and obtain 139g of 0.73mol 3-dimethylaminomethylene-1,1 difluoro- 2,4-pentanedione, yield 80%.
[0051] Add 10ml of methylhydrazine to 85ml of dichloromethane and 14ml of distilled aqueous solution, cool the mixture to -20°C, control the temperature within the range of -25°C to -17.6°C, and add 33g of 0.17mol 3- Dimethylaminomethylene-1,1-difluoro-2,4-pentanedione with 90 ml of dichloromethane. After the addition, 170 mL o...
Embodiment 2
[0054] 102 ml of 1-dimethylamino-1-buten-3-one was added to a solution of 500 ml of dichloromethane and 254 ml of triethylamine, the mixture was cooled to -5°C, and then 86 ml of difluoroethylene was added to the mixture within 90 minutes Acetyl fluoride. After the addition was complete, the temperature of the reactants was kept at 5°C, and the mixture was continued to react for half an hour. Extract with 500ml of water, then extract the organic matter in the water with 500ml of dichloromethane, combine the organic phases, distill off the dichloromethane organic solvent under reduced pressure, and distill to obtain 122g of 3-dimethylaminomethylene-1,1 difluoro-2,4 -Pentanedione, yield 70%.
[0055] Add 10ml of methylhydrazine to 85ml of dichloromethane and 14ml of distilled aqueous solution, cool the mixture to -10°C, control the temperature within the range of -15°C to -5°C, and add 33ml of 3-dimethylamino to the mixture within 20 minutes Methylene-1,1-difluoro-2,4-pentaned...
Embodiment 3
[0058] 102ml of 1-dimethylamino-1-buten-3-one was added to 500ml of dichloromethane and 254ml of triethylamine solution, the mixture was cooled to -10°C, and then 100ml of difluoroethylene was added to the mixture within 90 minutes Acetyl fluoride. After the addition was complete, the temperature of the reactants was kept at 0°C, and the mixture was continued to react for half an hour. Extract with 500ml of water, then extract the organic matter in the water with 500ml of dichloromethane, combine the organic phases, distill off the dichloromethane organic solvent under reduced pressure, and distill to obtain 138g of 3-dimethylaminomethylene-1,1 difluoro-2,4 -Pentanedione, yield 79%.
[0059]Add 20ml of methylhydrazine to 85ml of dichloromethane and 14ml of distilled aqueous solution, cool the mixture to -20°C, control the temperature within the range of -25°C to -17.6°C, add 33ml of 3-dimethylamino to the mixture within 30 minutes Methylene-1,1-difluoro-2,4-pentanedione with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com