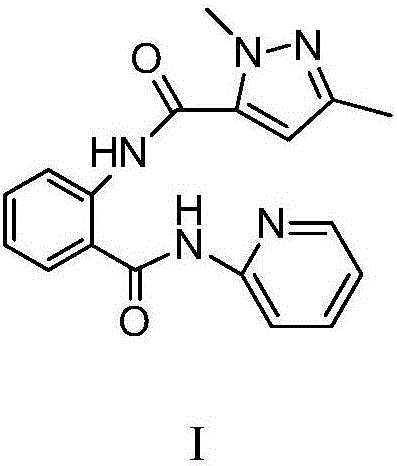
N-(2-pyridyl)-2-(2,4-dimethyl parazole formamido) benzamide as well as preparation thereof and use thereof
A technology of dimethylpyrazole carboxamide and benzamide, applied in N-(2-pyridyl)-2-(2,4-dimethylpyrazole carboxamide) benzamide and its preparation It can solve the problems of decreased pesticide use efficiency and easy resistance, and achieve the effect of outstanding antibacterial activity and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
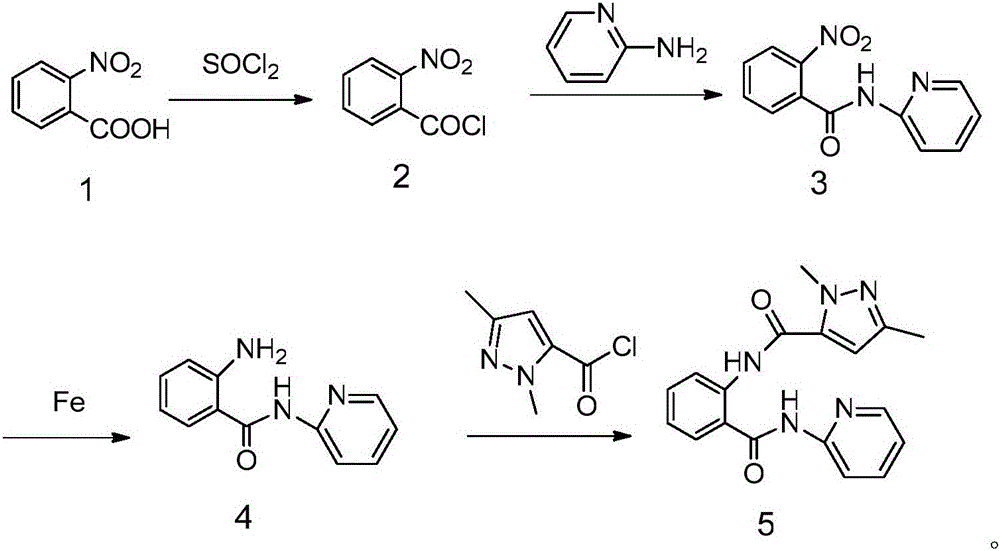
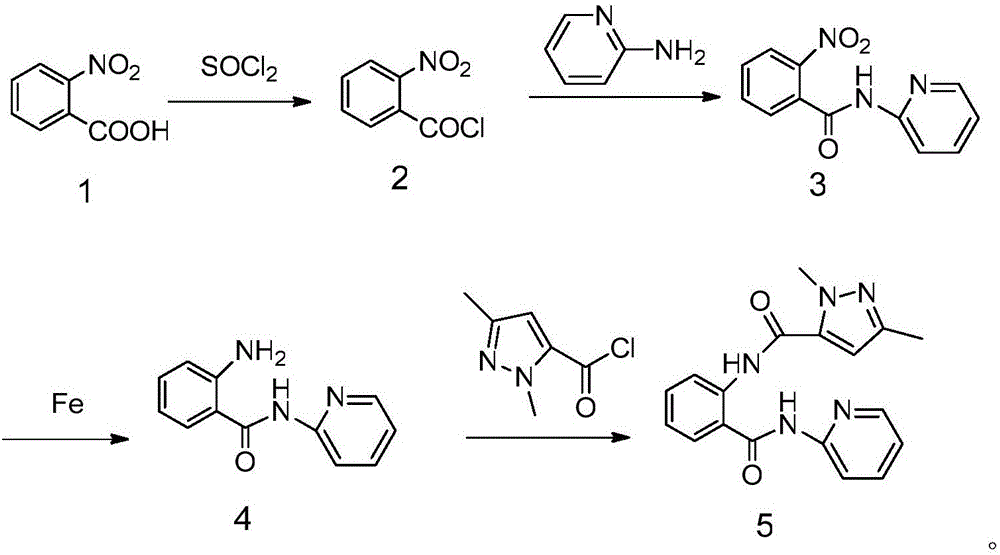
Examples
Embodiment 1
[0033] Synthesis of 2-nitro-N-(2-pyridyl)benzamide:
[0034] Add 4.18g (0.025mol) of o-nitrobenzoic acid and 10mL (0.14mol) of thionyl chloride into a 50mL flask, heat to reflux for 6h, evaporate the solvent under reduced pressure to obtain o-nitrobenzoyl chloride for later use. Add 30mL of dehydrated dichloromethane, 5g (0.05mol) of triethylamine and 2.35g (0.025mol) of 2-aminopyridine into a 50mL three-necked flask, and dissolve o-nitrobenzoyl chloride into the dehydrated dichloromethane solution under stirring at 0°C Slowly add it dropwise into a three-necked flask, stir the reaction, and follow the reaction by TLC. After the reaction is completed, dilute with 30 mL of dichloromethane and 25 mL of water, extract the aqueous layer with dehydrated dichloromethane (20 mL×3), combine the organic layers, and wash the organic layer with 1.2 mol / L NaHCO3 aqueous solution was washed until neutral, then washed with saturated brine, dried with anhydrous MgSO4, filtered, and precipita...
Embodiment 2
[0036] Synthesis of 2-nitro-N-(2-pyridyl)benzamide:
[0037] Add 4.18g (0.025mol) of o-nitrobenzoic acid and 20mL (0.28mol) of thionyl chloride into a 50mL flask, heat to reflux for 3h, and distill off excess thionyl chloride under reduced pressure to obtain o-nitrobenzoyl chloride spare. Add 15mL of anhydrous THF, 5g (0.05mol) of triethylamine and 2.35g (0.025mol) of 2-aminopyridine into a 50mL three-necked flask, and slowly dissolve the solution of o-nitrobenzoyl chloride in anhydrous THF under stirring at 0°C. Add dropwise into a three-necked flask, stir the reaction, follow the reaction by TLC, dilute with 30mL ethyl acetate and 25mL water after the reaction is complete, extract the aqueous layer with ethyl acetate (20mL×2), combine the organic layers, and wash the organic layer with 1.2mol / LNaHCO 3 Wash with aqueous solution to neutrality, then wash with saturated brine, anhydrous Na 2 SO 4 Dry, filter, and precipitate under reduced pressure to obtain a crude product...
Embodiment 3
[0039] Synthesis of 2-nitro-N-(2-pyridyl)benzamide:
[0040] Add 4.18g (0.025mol) of o-nitrobenzoic acid and 10mL (0.14mol) of thionyl chloride into a 50mL flask, heat to reflux for 6h, evaporate the solvent under reduced pressure to obtain o-nitrobenzoyl chloride for later use. Add 15mL of anhydrous THF, 5g (0.05mol) triethylamine and 2.35g (0.025mol) of 2-aminopyridine into a 50mL three-necked flask, and slowly drop the anhydrous THF solution of o-nitrobenzoyl chloride into the three-necked flask while stirring at 0°C. In the flask, stir the reaction, follow the reaction by TLC, after the reaction is completed, dilute with 30mL ethyl acetate and 25mL water, extract the aqueous layer with ethyl acetate (20mL×2), combine the organic layers, and use 1.2mol / L NaHCO for the organic layer 3 Wash with aqueous solution to neutrality, then wash with saturated brine, anhydrous Na 2 SO 4 Drying, filtration, and precipitation under reduced pressure gave the crude product, which was re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com