Trifluoromethyl pyrazole derivative and applications thereof
A technology of trifluoromethylpyrazole and fluoromethylpyrazole, which is applied in the field of new trifluoromethylpyrazole derivatives and applications, can solve dependence, the range of synthetic substrates and the lack of wide application, and triazene There are few problems in the application of intermediates, etc., to achieve the effect of environmental protection operation and simple and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
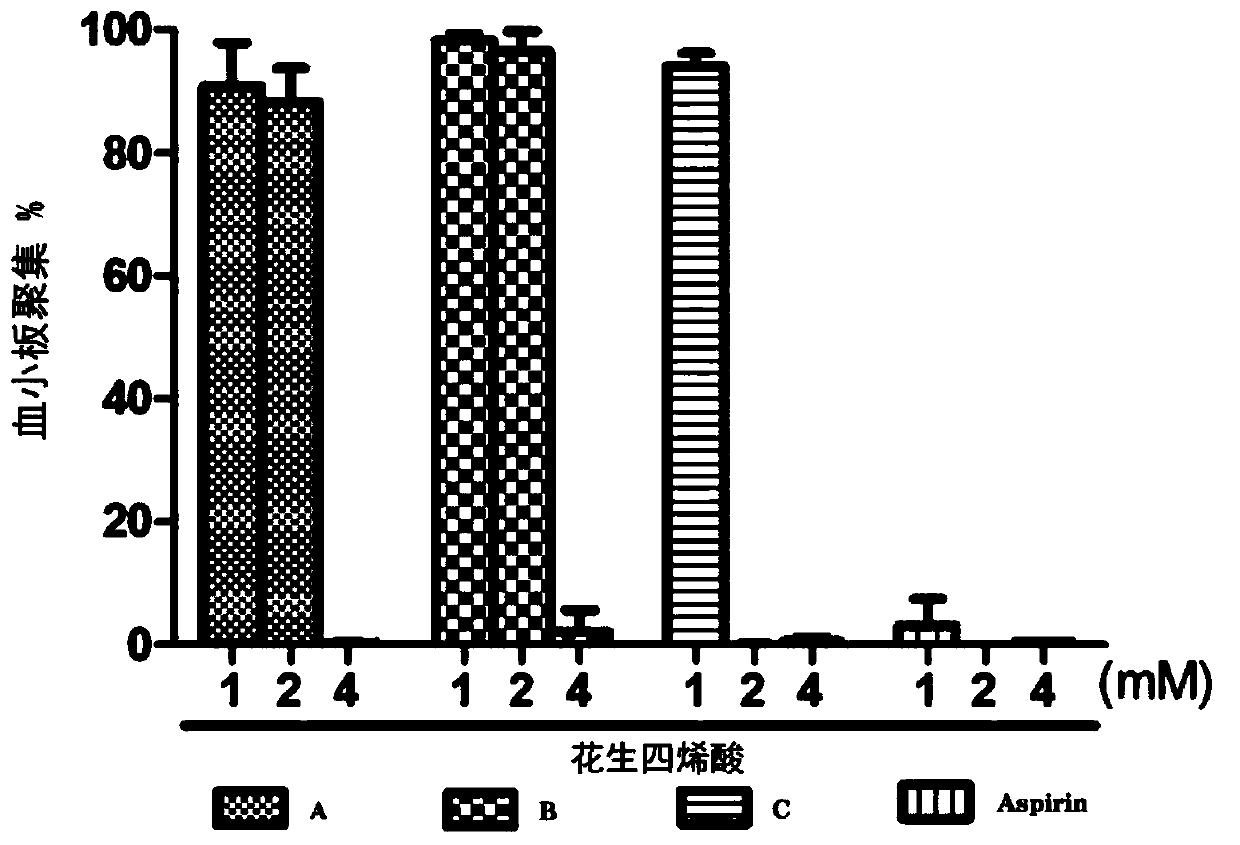
Image
Examples
Embodiment 1
[0045] Example 1: Synthesis of 5-phenyl-3-trifluoromethyl-1H-pyrazole
[0046]
[0047] In the presence of DBU (0.06mmol, 0.2eq) as Lewis base, 1,4-dioxane (0.4mL) was added as solvent, phenylacetylene (0.3mmol, 1.0eq) and CF 3 CHN 2(1.2mmol, 4.0 equivalents, 1.5mol / L toluene) was reacted at 80°C for 12h, and the solvent was evaporated off after the reaction to obtain a light yellow solid 5-phenyl-3-trifluoromethyl-1H-pyrazole, the yield 97%.
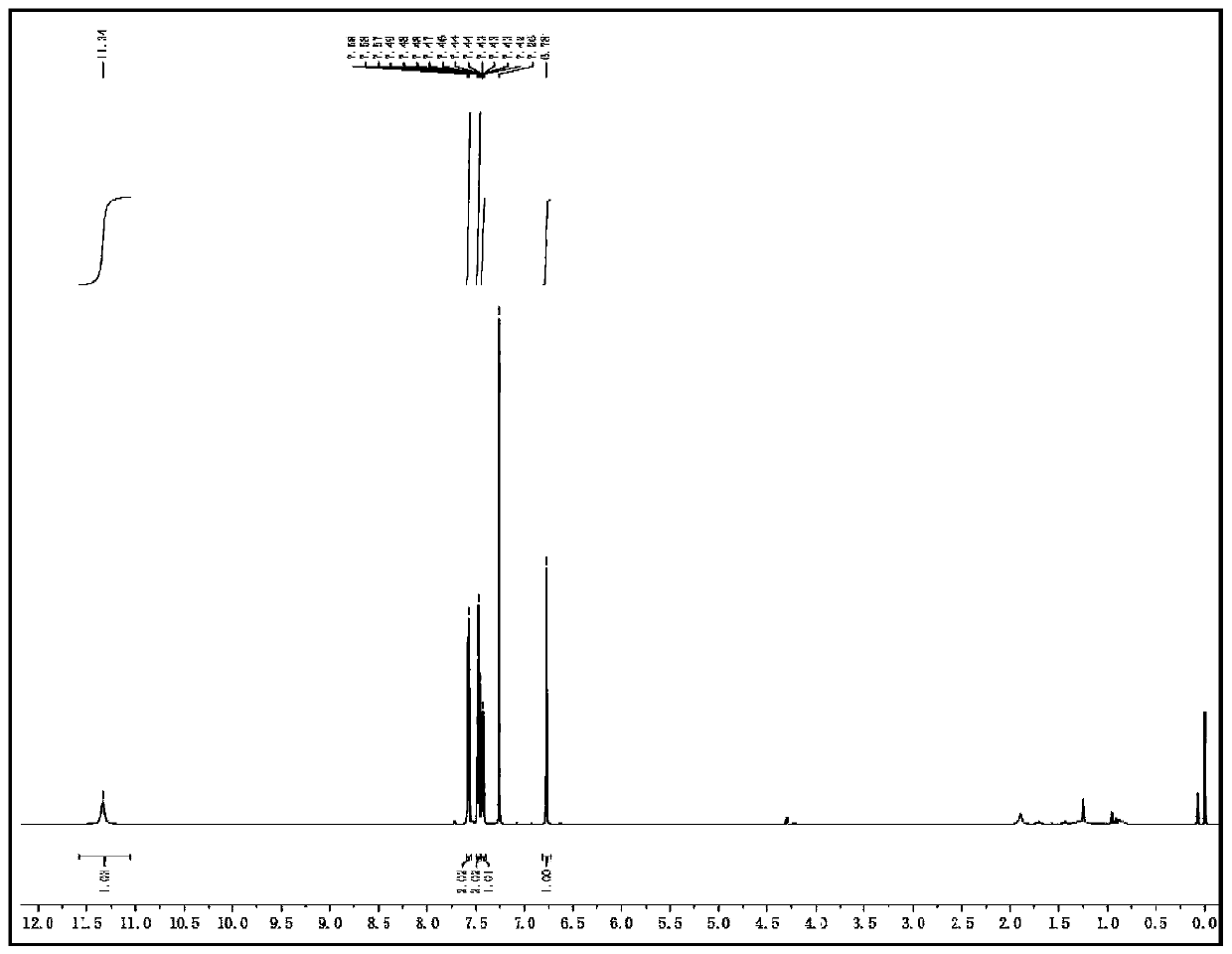
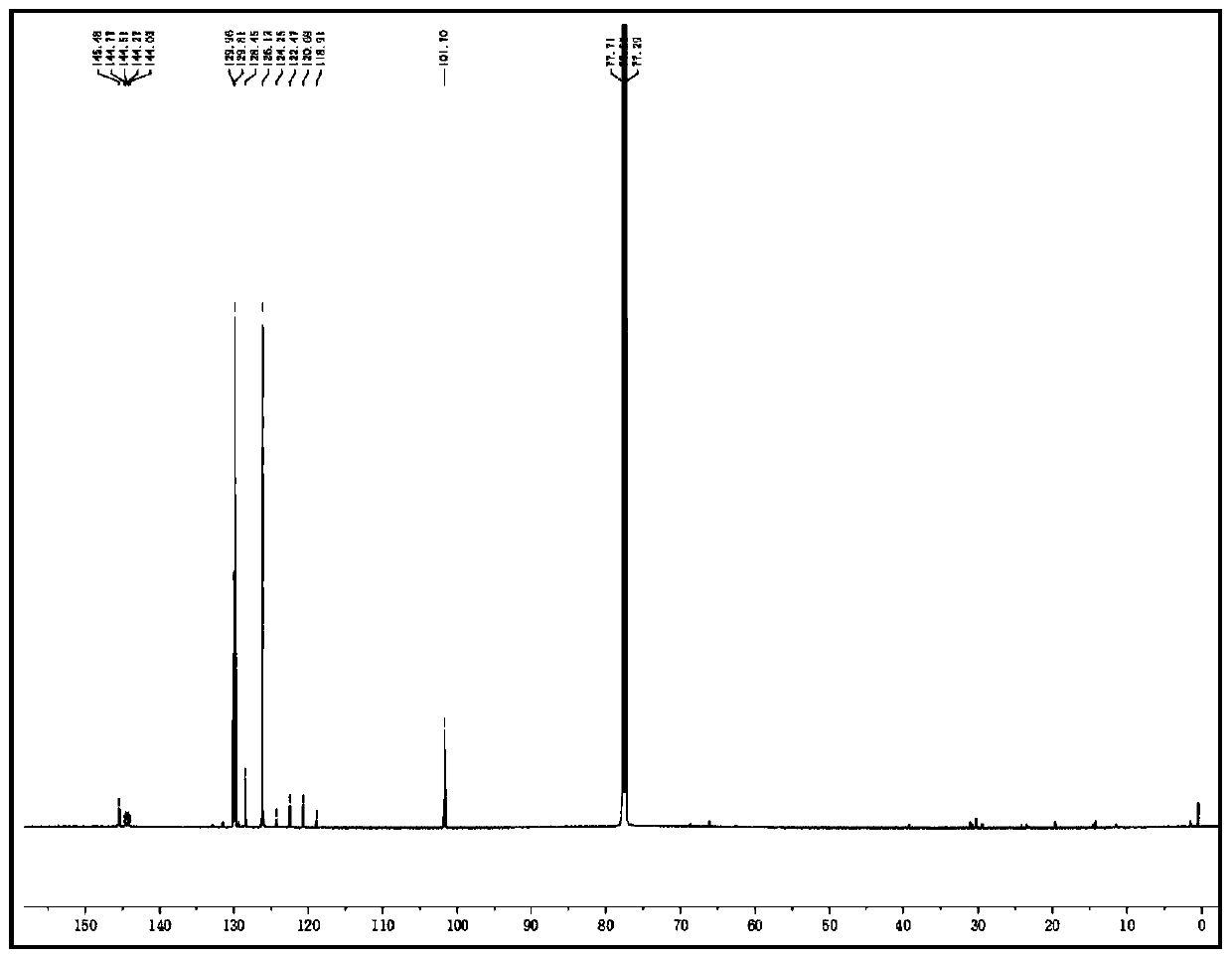
[0048] 1H NMR (600MHz, CDCl3): δ=11.34(s,1H), 7.58–7.57(m,2H), 7.49–7.46(m,2H), 7.44–7.42(m,1H), 6.78(s,1H) ppm.13C NMR (151MHz, CDCl3): δ=145.5, 144.4 (q, JC-F=36.2Hz), 1230.0, 129.8, 128.45, 126.1, 121.6 (q, JC-F=238.7Hz), 101.7ppm.HRMS (ESI): calcd for C10H8N2F3[M +H]+: 213.0634, found 213.0631
[0049] 1H-NMR spectrum and 13C-NMR spectrum as figure 1 , 2 As shown, it was detected by a nuclear magnetic resonance spectrometer (Bruker VNMRS600).
Embodiment 2
[0050] Example 2: Synthesis of 3-(3-(trifluoromethyl)-1H-pyrazol-5-yl)pyridine
[0051]
[0052] In the presence of DBU (0.06mmol, 0.2eq) as Lewis base, 1,4-dioxane (0.4mL) was added as solvent, 3-alkynyl-pyridine (0.3mmol, 1.0eq) and CF 3 CHN 2 (1.2mmol, 4.0 equivalents, 1.5mol / L toluene) was reacted at 80°C for 16h, and after the reaction was completed, the solvent was evaporated to obtain a light yellow solid 3-(3-(trifluoromethyl)-1H-pyrazole-5- Base) pyridine, yield 96%.
[0053] 1H NMR (600MHz, MeOD): δ = 8.95 (d, J = 1.2Hz, 1H), 8.58–8.57 (m, 1H), 8.19 (d, J = 7.9Hz, 1H), 7.55 (dd, J = 7.9 ,4.9Hz,1H),7.13(s,1H)ppm.13C NMR(151MHz,MeOD):δ=150.4,147.4,145.1,142.7(q,JC-F=32.6 Hz),135.3,126.8,125.8,122.8 (q, JC-F=267.2Hz), 103.0ppm.1HRMS(ESI): calcd for C9H5N3F3[M-H]-:212.0441, found212.0438.
[0054] 1H-NMR spectrum and 13C-NMR spectrum as image 3 , 4 shown.
Embodiment 3
[0055] Example 3: Synthesis of ethyl 5-phenyl-3-trifluoromethyl-1H-pyrazole-4-carboxylate
[0056]
[0057] In the presence of DBU (0.06mmol, 0.2eq) as Lewis base, 1,4-dioxane (0.4mL) was added as solvent, ethyl phenylpropiolate (0.3mmol, 1.0eq) and CF 3 CHN 2 (1.2mmol, 4.0 equivalents, 1.5mol / L toluene) was reacted at 80°C for 20h, and the solvent was evaporated off after the reaction to obtain a white solid 5-phenyl-3-trifluoromethyl-1H-pyrazole-4-carboxylate Acetate ethyl ester, yield 82%.
[0058] 1H NMR (600MHz, CDCl3): δ=11.34(s, 1H), 7.53(dd, J=5.2, 3.1Hz, 2H), 7.48–7.43(m, 3H), 4.25(q, J=7.1Hz, 2H ), 1.24(t,J=7.1Hz,3H)ppm.
[0059] 13C NMR (151MHz, CDCl3): δ=162.0, 148.9, 143.7 (q, JC-F=37.8Hz), 130.8, 129.5, 129.1, 127.6, 121.0 (q, JC-F=268.8Hz), 109.94, 61.59, 14.18.ppm.1HRMS(ESI):calcd for C13H12O2N2F3[M+H]+:285.0845, found 285.0842.
[0060] 1H-NMR spectrum and 13C-NMR spectrum as Figure 5 , 6 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com