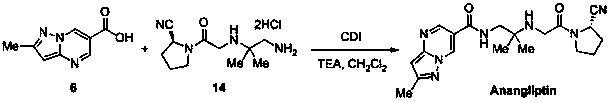
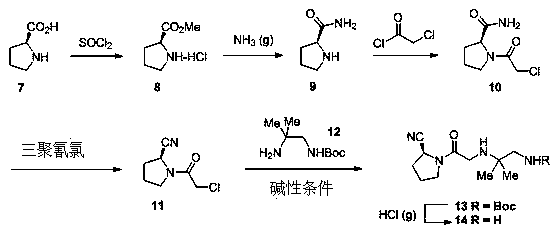
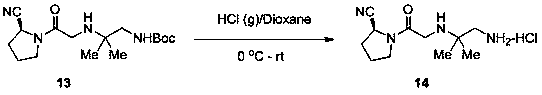Synthesis method of anagliptin
A synthetic method and intermediate technology, which is applied in the field of synthesis of anagliptin (Anagliptin) raw materials, can solve the problems of high price, and achieve the effect of cheap raw materials, mild reaction conditions, and convenient post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0023] 1. Preparation of 1,1,1-trichloro-4-ethoxy-3-vinyl-2-one (2)
[0024]
[0025] Vinyl ether (20kg, 277mol, 2.0eq.) was placed in a 100L reactor, cooled to 0°C, and trichloroacetyl chloride (25kg, 139mol, 1.0eq.) was slowly added. After the dropwise addition was completed, the temperature was raised slowly and kept at room temperature for 9 hours until the reaction was completed. First remove the solvent under reduced pressure, then heat up to 100°C and distill under reduced pressure until there is no obvious fraction, and finally heat up to 120°C and then distill under high vacuum to obtain the product 2 It is light yellow liquid (25kg, yield 82%). 1 H-NMR (400MHz, CDCl 3 ) δ 7.81(d,1H, J =12.0Hz), 6.96(d, 1H, J=12.0Hz), 4.05(q, 2H, J=7.2Hz), 1.34(t, 3H, J=7.2Hz)ppm.
[0026] 2. Preparation of ethyl 3,3-diethoxypropionate (3)
[0027]
[0028] Add absolute ethanol (30kg) and K2CO3 (1.6kg, 11.6mol) into a 100L reactor, then slowly add compound 2 (25kg, 115mol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com