Preparation method of lorlatinib
A technology of lorlatinib and compounds, applied in the field of preparation of lorlatinib, which can solve problems such as unfavorable industrial production, reduced synthesis efficiency of lorlatinib, and limitation of application of lorlatinib
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0082] The preparation method of lorlatinib
[0083] The present invention provides a kind of preparation method of lorlatinib, described method comprises the steps:
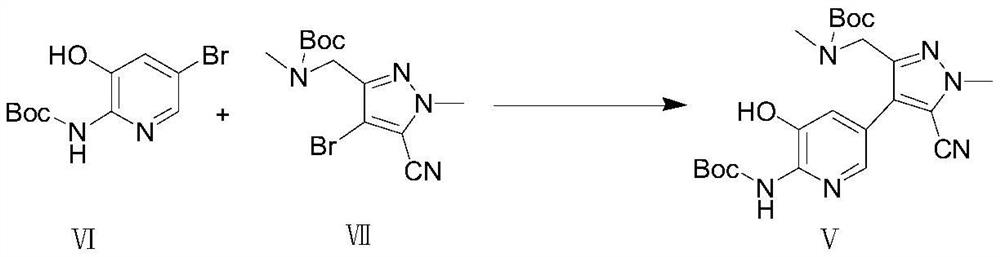
[0084] (1) In the first solvent, under the first basic reagent, the compound of formula VI and the compound of formula VII undergo a coupling reaction under the catalysis of a palladium catalyst to obtain the compound of formula V;
[0085]
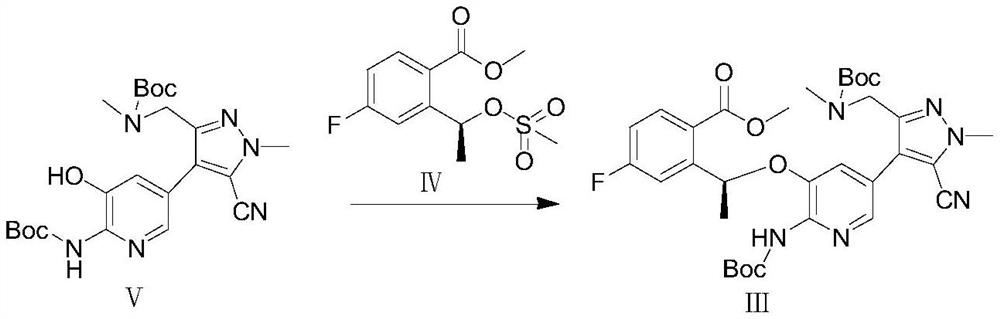
[0086] (2) In the second solvent, under the second basic reagent, the compound of formula V and the compound of formula IV undergo Williamson reaction to obtain the compound of formula III;
[0087]
[0088] (3) the compound of formula III undergoes hydrolysis reaction and acidolysis reaction successively to obtain the compound of formula II;
[0089]
[0090] (4) the compound of formula II is subjected to condensation reaction to obtain the compound of formula I;
[0091]
[0092] The preparation of formula V compound
[0093] The present invention provides ...
Embodiment 1
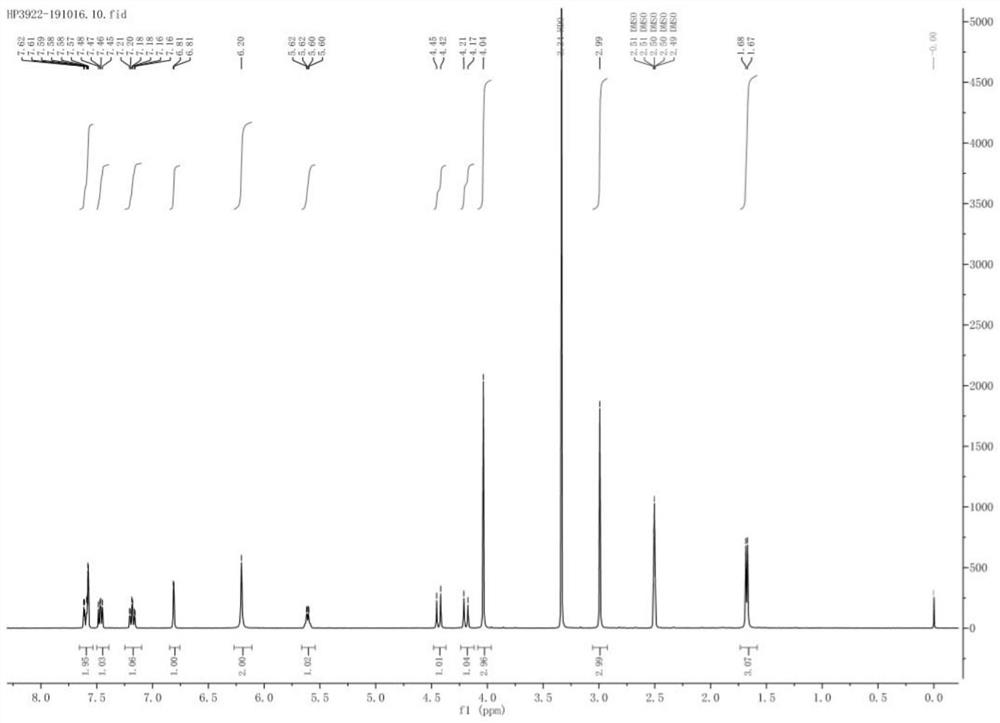
[0170] Preparation of compound (Ⅴ)
[0171]
[0172] Add 28.8g (0.1mol) of compound (Ⅵ) and 32.9g (0.1mol) of compound (Ⅶ), 400ml of 1,4-dioxane and 100ml of water into a 1L reaction flask, pass through nitrogen protection, add 0.3g of 1,1 '-bis(diphenylphosphino)ferrocenepalladium dichloride and 34.6g (0.25mol) of potassium carbonate were heated up to 70°C and reacted for 3h. Cool down to room temperature, remove insoluble matter by filtration, concentrate the filtrate under reduced pressure, add ethyl acetate for extraction, wash the ethyl acetate layer with water, and concentrate the ethyl acetate layer to dryness to obtain 40.3 g of a yellow solid with a molar yield of 88%, MS (ESI ): [M]=458.51.
Embodiment 2
[0174] Preparation of Compound (Ⅲ)
[0175]
[0176] Add 27.5g (0.06mol) of compound (Ⅴ), 400ml of acetone, 16.6g (0.12mol) of powdered potassium carbonate, 17.4g (0.063mol) of compound (Ⅳ) to a 1L reaction flask, raise the temperature to 55°C, react for 18h, and cool down to room temperature, filter. The filtrate was concentrated to dryness, 80ml of n-hexane and 20ml of ethyl acetate were added, stirred, filtered, and dried to obtain 36.4g of compound (Ⅲ), with a molar yield of 95%. MS (ESI): [M] = 638.69.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com